Linnaeus Therapeutics to Present Positive LNS8801 Results in Metastatic Melanoma at ESMO 2024
Linnaeus Therapeutics, Inc. (Linnaeus), a privately owned biopharma company in the clinical stage that specializes in developing and commercializing new small-molecule cancer treatments, revealed clinical findings at the 2024 ESMO Annual Meeting. The data pertains to the dose-expansion cohort of LNS8801 as a single-agent treatment in patients with metastatic cutaneous melanoma who were unable to undergo immunotherapy because of previous immune-related adverse reactions.
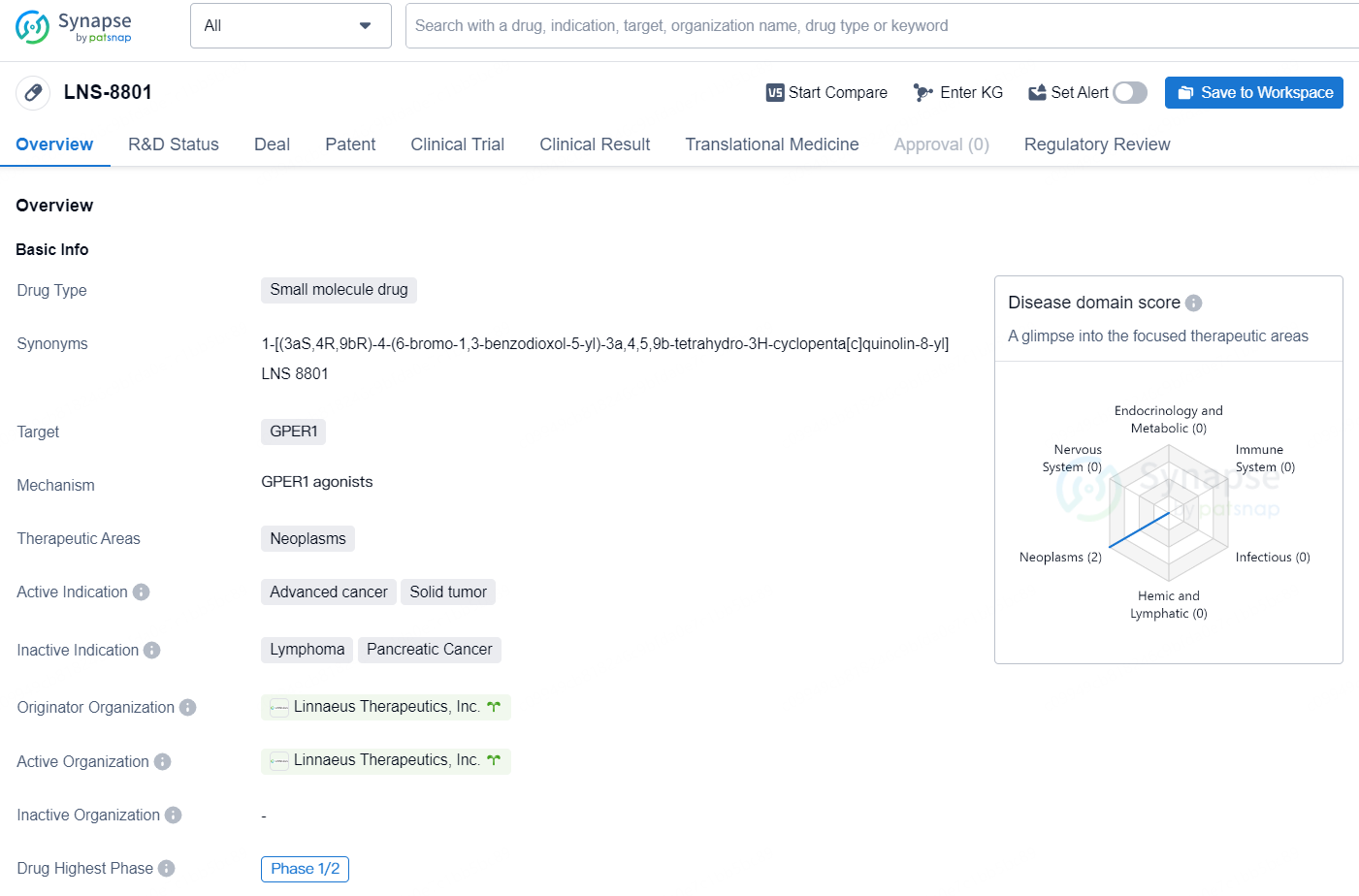
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The poster is titled “Efficacy of LNS8801 in melanoma patients with prior immune-related adverse events from immune-checkpoint inhibitors” (Abstract 1137P).
Administration of LNS8801 on its own was manageable without unexpected toxicities and showcased promising antitumor effects in individuals with metastatic cutaneous melanoma who previously experienced severe immune-related adverse events from immune checkpoint inhibitor therapies. LNS8801 monotherapy led to a 100% disease control rate in biomarker-positive patients, with preliminary progression-free survival extending beyond 6 months. Notably, one patient has remained on treatment for over 4.5 years, with resolution of all soft-tissue lesions and no active disease detected in the remaining bone-associated target lesion or other areas.
“We are thrilled to present these findings at ESMO,” stated Patrick Mooney, MD, CEO of Linnaeus. “The results from this research illustrate that LNS8801 is extremely safe, well tolerated, and shows very promising efficacy. Since patients with prior irAEs are usually barred from further immunotherapy, these individuals represent a significant unmet need. We are eager to gather more data from this patient group and in unresectable, treatment-refractory melanoma.”
Linnaeus plans to start a randomized controlled clinical trial in unresectable, treatment-refractory cutaneous melanoma in the fourth quarter of this year. This trial will assign 135 biomarker-positive patients to receive either LNS8801 monotherapy, LNS8801 combined with pembrolizumab, or therapy chosen by the physician. The study will evaluate median progression-free survival and overall survival among the groups.
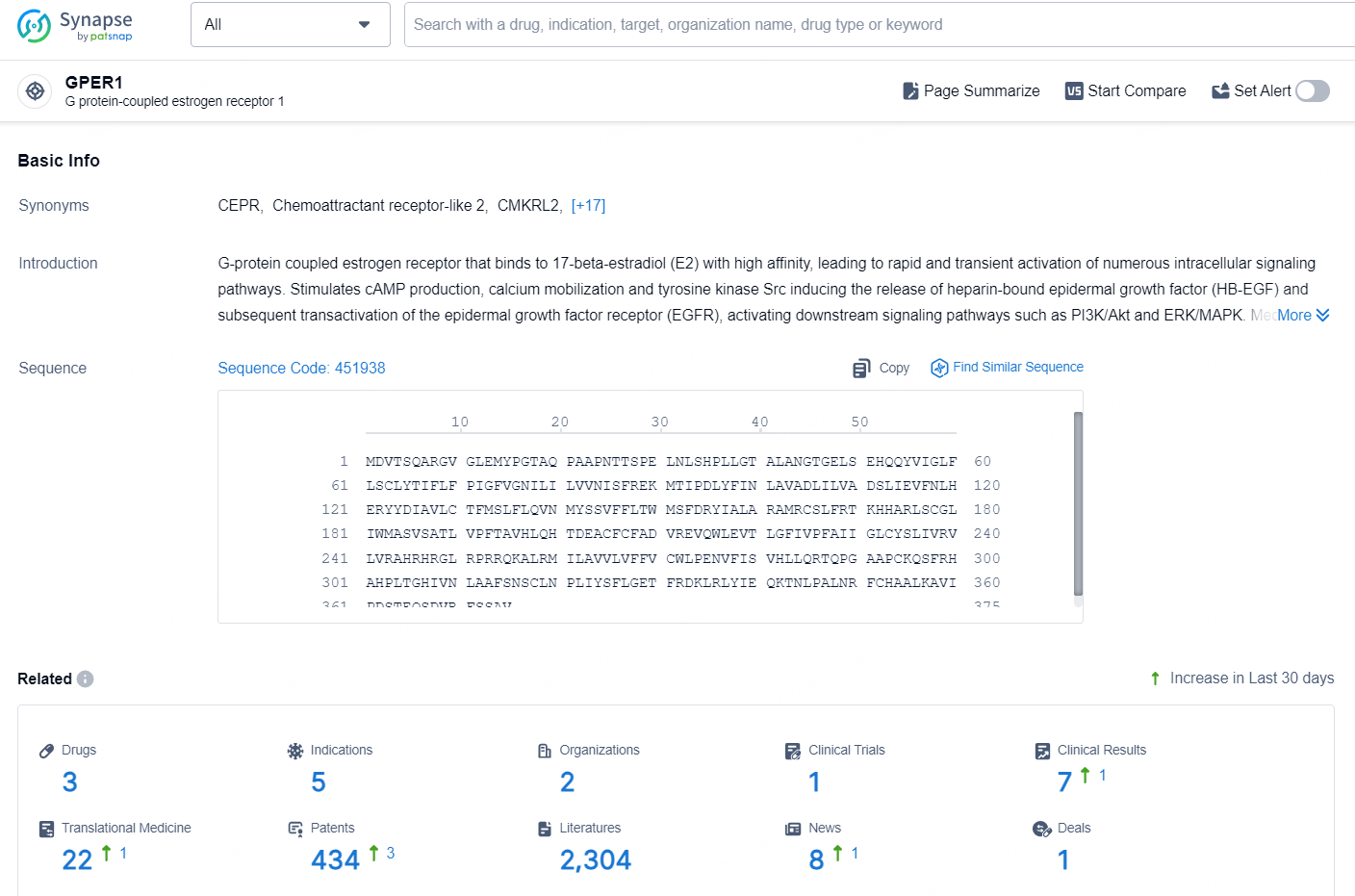
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 3 investigational drugs for the GPER1 target, including 5 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 434 patents.
LNS-8801 is a small molecule drug developed by Linnaeus Therapeutics, Inc., designed to target GPER1 in the treatment of neoplasms, specifically advanced cancer and solid tumors. The drug is currently in Phase 1/2 of clinical development, indicating that it is undergoing testing on a small scale to assess its safety and efficacy in treating these conditions.