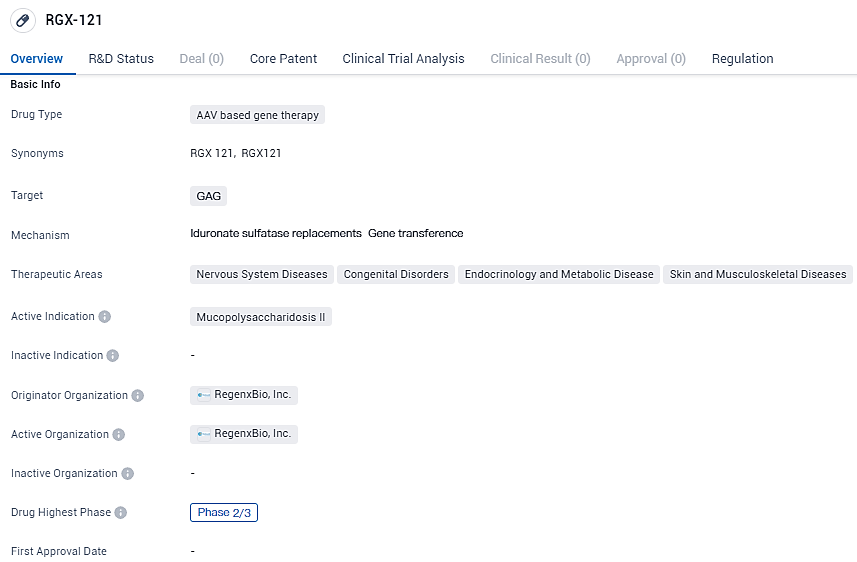
REGENXBIO Hits Key Goal in RGX-121 Study for MPS II
REGENXBIO Inc. disclosed preliminary findings from the initial and intermediate stages of the CAMPSIITE study on RGX-121, formulated for young individuals no older than five years, with a confirmed diagnosis of Mucopolysaccharidosis Type II, commonly referred to as Hunter syndrome. The data highlighted that the crucial phase of this investigation satisfied its foremost objective with a significant level of statistical prominence.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Emerging evidence from the pivotal study indicates that RGX-121 has the capability to alter the progression of Hunter syndrome by replenishing the deficient gene in affected male patients, with a strong likelihood of enhancing crucial cognitive functions in individuals afflicted by this severe condition," stated Kenneth T. Mills, the chief executive of REGENXBIO.
Mills expressed his enthusiasm regarding these breakthroughs and the company's rapid progress towards submitting the BLA within the current year. He mentioned that findings from the CAMPSIITE study were presented to FDA officials, who acknowledged the possibility of an expedited approval based on the comprehensive data review.
Dr. Harmatz also showed optimism, commenting on the absence of current therapies for the deadly CNS manifestation of MPS II and expressing encouragement over the preliminary results from RGX-121's pivotal study. Dr. Harmatz noted that a single administration of this gene therapy could notably alter disease progression, potentially eliminating the need for ongoing enzyme replacement therapies, signifying a substantial advancement.
After a late-2023 RMAT consultation with the FDA, REGENXBIO is moving ahead with their stratagem to seek accelerated endorsement utilizing the surrogate marker, CSF levels of D2S6. The firm is finalizing necessary measures to lodge a BLA in the latter part of 2024. With an anticipated expedited evaluation, the BLA's approval could lead to the acquisition of a Rare Pediatric Disease Priority Review Voucher by 2025.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
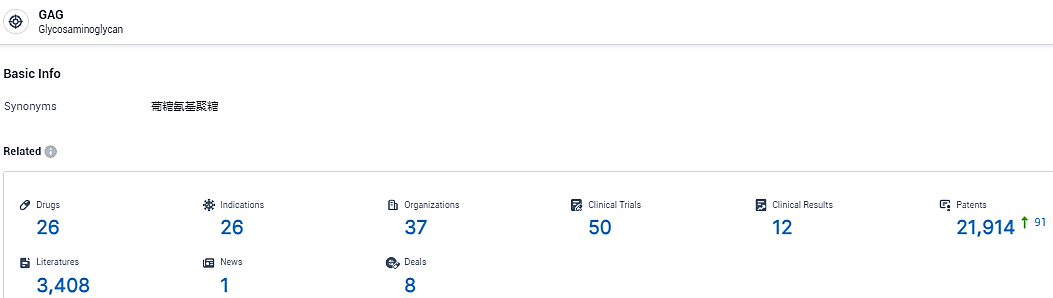
According to the data provided by the Synapse Database, As of February 19, 2024, there are 26 investigational drugs for the GAG target, including 26 indications, 37 R&D institutions involved, with related clinical trials reaching 50, and as many as 21914 patents.
RGX-121 targets GAG and is primarily focused on treating Mucopolysaccharidosis II, a rare genetic disorder. RGX-121 has received Orphan Drug Product, Rare Pediatric Disease, Fast Track and Regenerative Medicine Advanced Therapy designations from the U.S. Food and Drug Administration and advanced therapy medicinal products classification from the European Medicines Agency. With its advanced clinical development stage and regulatory designations, RGX-121 shows promise in addressing unmet medical needs in the field of biomedicine.