Transposon's Phase 2 Results on TPN-101 for C9orf72-Linked ALS and FTD
Transposon Therapeutics, a biotech firm focusing on creating new oral treatments for neurodegenerative and aging-associated illnesses such as Alzheimer’s disease, revealed the conclusive findings from its Phase 2 trial of TPN-101 in individuals suffering from amyotrophic lateral sclerosis and/or frontotemporal dementia linked to the hexanucleotide repeat expansion in the C9orf72 gene (C9orf72-related ALS/FTD).
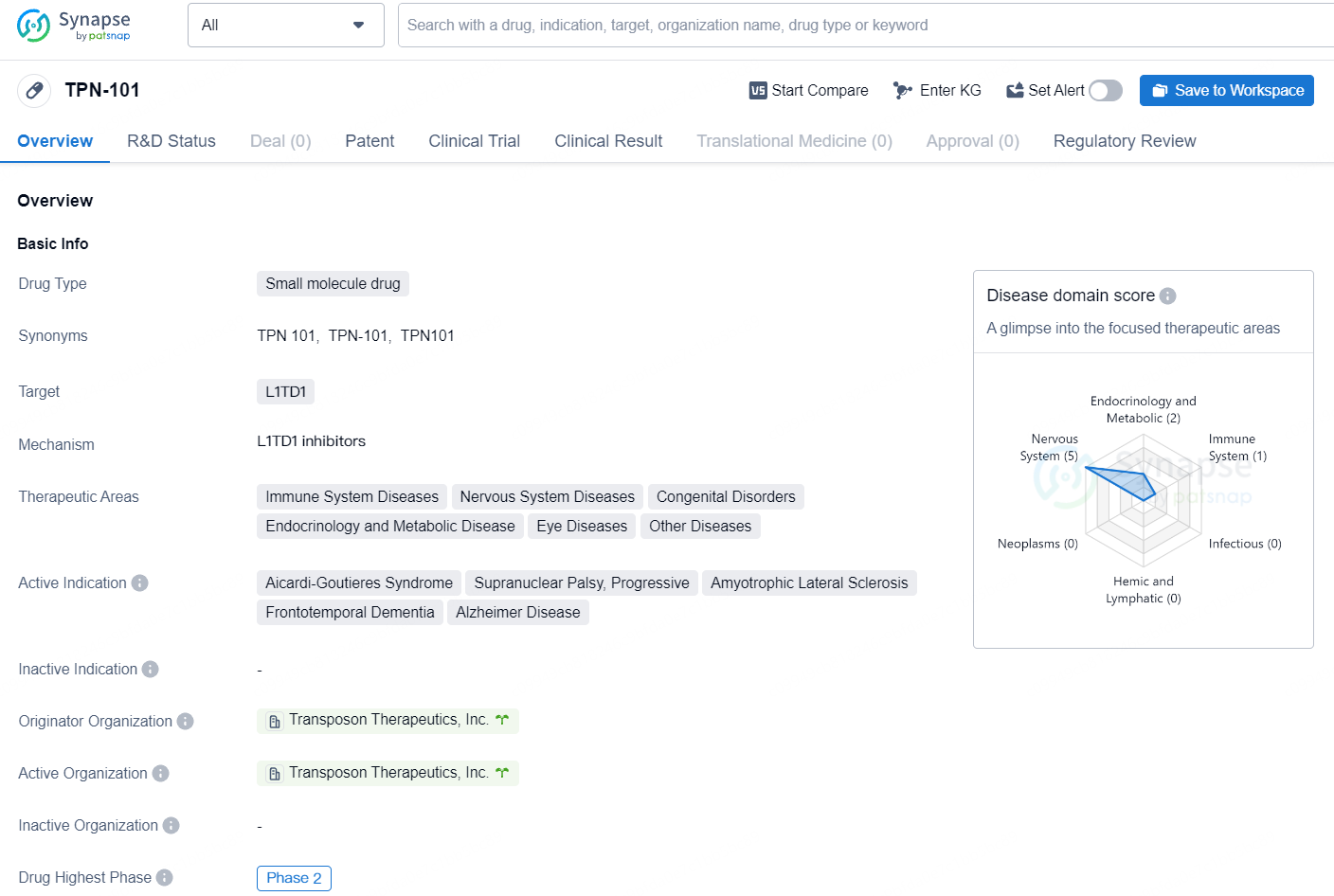
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The conclusive findings from this research reaffirmed the exemplary safety profile of TPN-101 and revealed clinical indicators of disease-modifying effects in patients with these conditions, supporting previously documented safety and therapeutic efficacy in individuals with Progressive Supranuclear Palsy. These outcomes align with the capability of TPN-101 to diminish neuroinflammation and neurodegeneration by inhibiting LINE-1 activity, a human-specific retrotransposon inadequately suppressed in many neurodegenerative disorders and aging.
Amyotrophic Lateral Sclerosis (ALS) is a progressively fatal neurodegenerative condition with an average survival span of two to three years. Respiratory failure remains the predominant cause of mortality in ALS patients. Vital Capacity (VC) is an objective measure of respiratory function that correlates significantly with survival in these individuals. After a 24-week treatment period, C9-ALS patients on TPN-101 exhibited about a 50% lesser decline in VC compared to the placebo group.
During the open-label phase, when participants initially on placebo were switched to TPN-101, the decline in VC over the following 24 weeks was less than half compared to their time on placebo, and it was comparable to the original TPN-101 group during the double-blind phase. Overall, the 48-week changes in both cohorts were lower than those predicted based on the natural history of similar study populations.
“The impact of TPN-101 across several key endpoints in this study is promising and signifies an important step towards a potential treatment for this dire condition,” stated Merit Cudkowicz, M.D., Chair of the Massachusetts General Hospital Department of Neurology, Julieanne Dorn Professor of Neurology at Harvard Medical School, and principal investigator of the Phase 2 study of TPN-101 for C9orf72-related ALS/FTD. “I am eager to advance the development of TPN-101 and its potential significance for individuals living with C9-ALS.”
In participants with C9-ALS, those receiving TPN-101 had reduced levels of neurofilament light chain (NfL) compared to the placebo group at the end of the double-blind period. NfL serves as a primary biomarker of neurodegeneration. NfL results at both Weeks 24 and 48 are in line with the findings from the company's Phase 2 study of TPN-101 for PSP treatment. TPN-101 also reduced levels of additional neurodegeneration and neuroinflammation biomarkers, including neurofilament heavy chain, interleukin 6, neopterin, and osteopontin.
"With very limited treatment options for ALS patients, and given these results showing significant benefits for patients treated with TPN-101 across various functional and biomarker measures, we plan to rapidly advance TPN-101 into a Phase 3 registration study for C9-ALS treatment," stated Dennis Podlesak, Chairman and CEO of Transposon. “Beyond ALS, we are committed to advancing TPN-101 for the treatment of PSP, Alzheimer’s disease, and other neurodegenerative and autoimmune disorders, aiming to provide new and innovative therapies that can markedly improve the lives of those battling these devastating diseases.”
TPN-101 specifically targets the LINE-1 reverse transcriptase that facilitates LINE-1 replication. LINE-1 elements belong to a class of retrotransposable elements uniquely capable in humans of replicating and migrating to new locations within the genome. When dysregulated, LINE-1 reverse transcriptase drives the overproduction of LINE-1 DNA, triggering innate immune responses that contribute to neurodegenerative, autoimmune, and aging-related disease pathology.
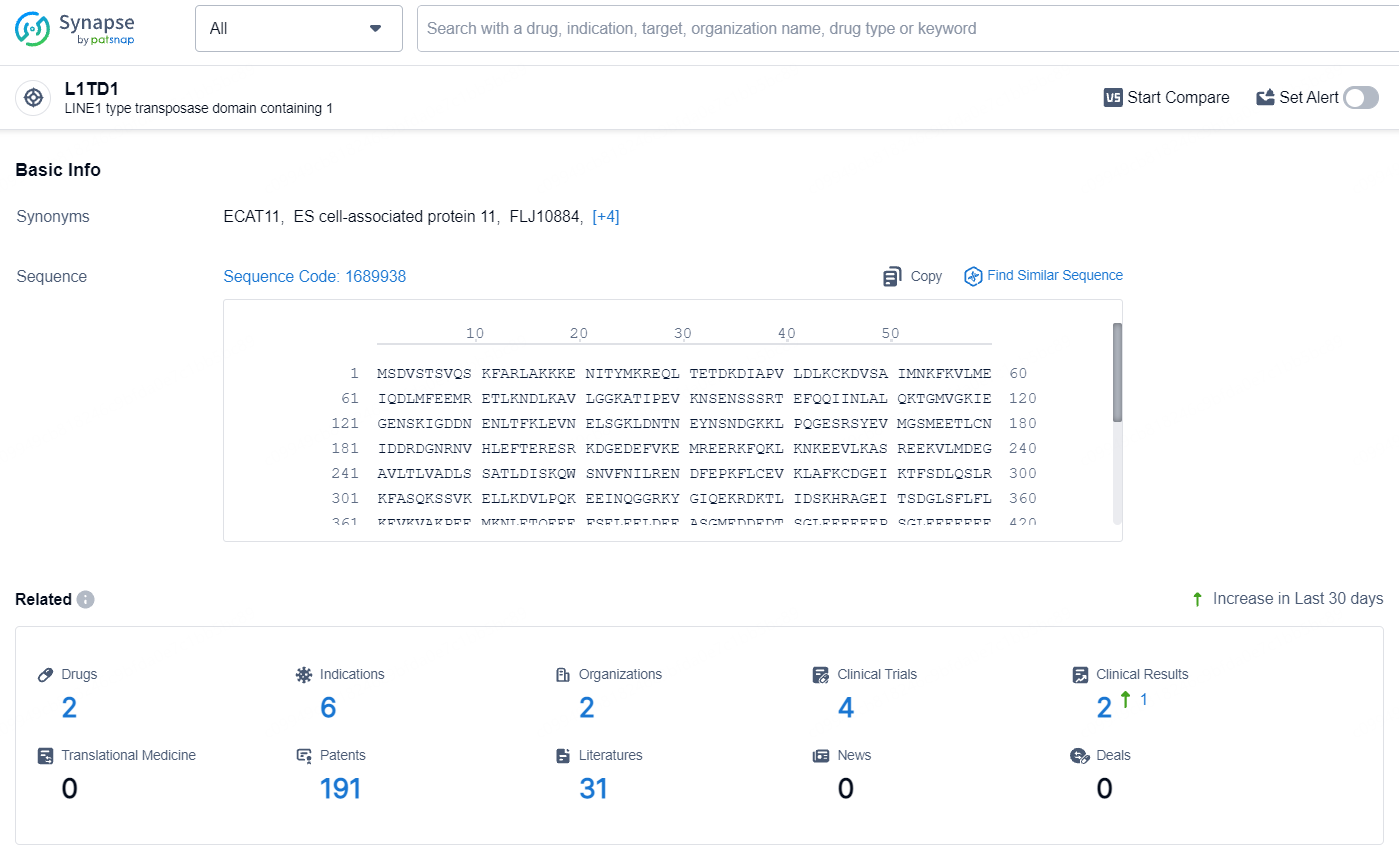
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 30, 2024, there are 2 investigational drugs for the L1TD1 targets, including 6 indications, 2 R&D institutions involved, with related clinical trials reaching 4, and as many as 191 patents.
TPN-101 presents an exciting opportunity within the pharmaceutical industry, particularly in the treatment of various neurological and genetic disorders. Its advancement to Phase 2 and Fast Track designation demonstrate both promise and progress in addressing unmet medical needs for patients with these conditions.