Sage Therapeutics and Biogen Present Phase 2 Results of SAGE-324 (BIIB124) for Essential Tremor
Sage Therapeutics, Inc. and Biogen Inc. disclosed the primary outcomes of the Phase 2 KINETIC 2 dose-range trial for the oral experimental medication SAGE-324 (BIIB124), aimed at treating essential tremor.
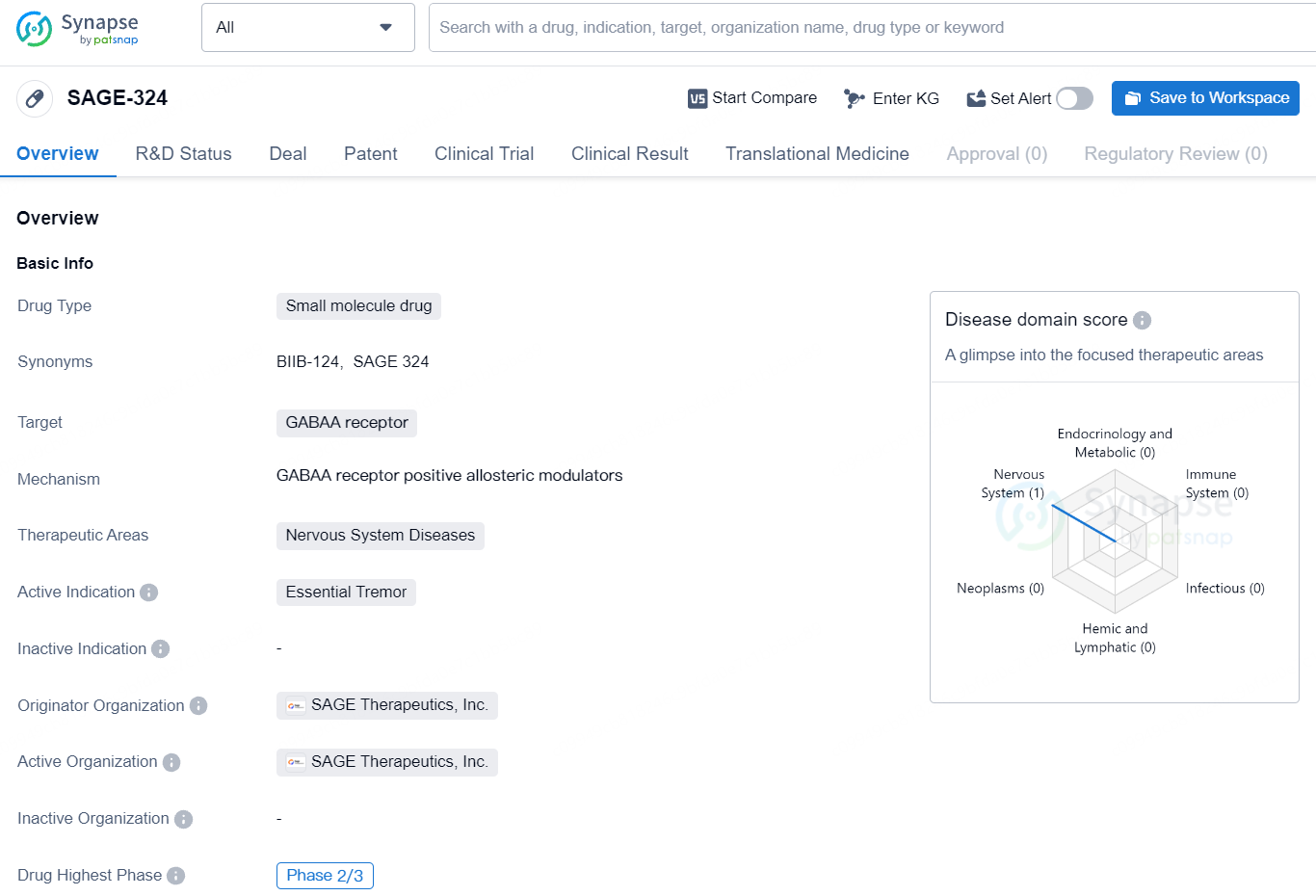
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The KINETIC 2 Study did not show a statistically significant dose-response relationship in changes from baseline to Day 91 on the primary endpoint, the Essential Tremor Rating Assessment Scale Performance Subscale Item 4 total score in participants with essential tremor (ET). Furthermore, no statistically significant differences were found between any dose of SAGE-324 and placebo in the change from baseline to Day 91 on the TETRAS PS Item 4 Total Score or the TETRAS Activities of Daily Living Composite Score.
In light of these outcomes, Sage and Biogen will terminate the ongoing open-label safety study of SAGE-324 in ET and have no plans for further clinical development of SAGE-324 in this condition. The companies are considering next possible steps for other potential uses.
“There has been minimal innovation in pharmacological treatments for essential tremor over the last 50 years, and those affected by this debilitating condition urgently need new treatment options. The results of the KINETIC 2 Study not supporting further development of SAGE-324 in ET are disappointing. We are thankful to the essential tremor community and study investigators for their contributions to this research,” said Laura Gault, MD, PhD, Chief Medical Officer, Sage Therapeutics. “Sage is committed to developing new treatments for people suffering from brain health disorders.”
“We would like to extend our gratitude to the study participants and researchers who enabled this important research. Despite the disappointing results, we believe that the findings contribute to the overall understanding of this debilitating condition and may guide future research and therapeutic approaches,” said Katherine Dawson, MD, Head of Therapeutics Development Unit, Biogen.
The KINETIC 2 Study was intended to assess the dose-response relationship of various doses of SAGE-324 on upper limb tremor. It also examined the safety and tolerability of SAGE-324. The primary outcome measure was the TETRAS PS Item 4 Total Score at Day 91, with the main analysis evaluating the dose-response relationship across SAGE-324 doses on this metric. Additional analyses looked at changes from baseline to Day 91 on the TETRAS PS Item 4 Total Score and the secondary endpoint, TETRAS ADL Composite Score, for each dose of SAGE-324 versus placebo.
SAGE-324 is an investigational oral neuroactive steroid GABAA receptor positive allosteric modulator. NAS GABAA receptor PAMs bind to both synaptic and extrasynaptic GABAA receptors, enhancing the inhibitory activity of the GABAergic system, which is the primary inhibitory neurotransmission system in the brain. GABA is the central inhibitory neurotransmitter in the central nervous system and is crucial for maintaining balanced neuronal activity in the brain. GABA dysregulation has been implicated in the pathophysiology of ET. The safety and efficacy of SAGE-324 have not yet been established.
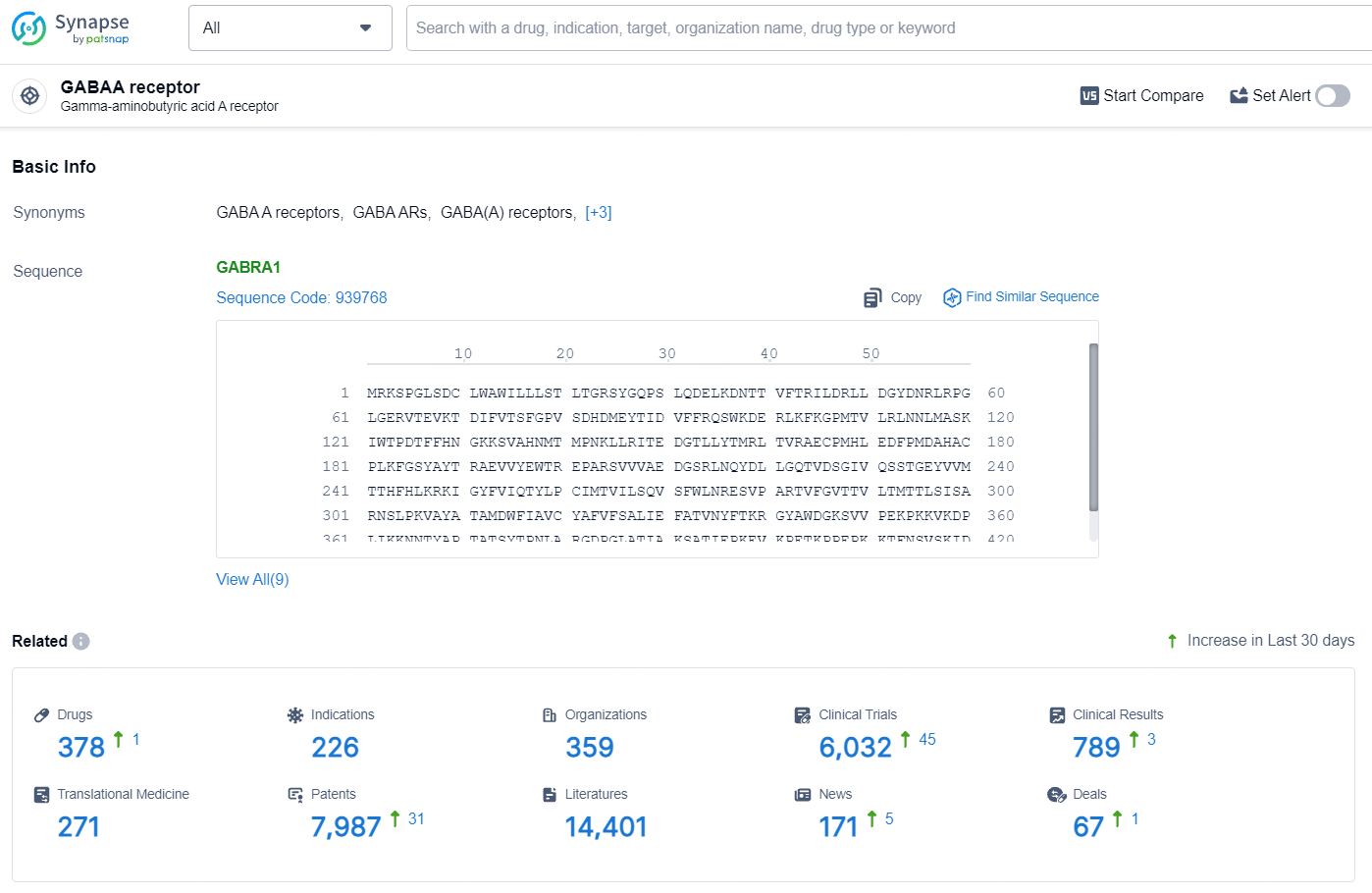
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 30, 2024, there are 378 investigational drugs for the GABAA target, including 226 indications, 359 R&D institutions involved, with related clinical trials reaching 6032, and as many as 7987 patents.
SAGE-324 represents a significant advancement in the development of potential therapeutics for nervous system diseases, particularly essential tremor. The drug's progress to Phase 2/3 indicates a promising future for individuals affected by this debilitating condition, though further clinical data and regulatory approvals will be necessary for its eventual market availability.