Elevation Oncology Reveals EO-3021 Strategy for Gastric Cancer Patients
Elevation Oncology, Inc., a pioneering company in the oncology field dedicated to discovering and developing targeted cancer treatments for various solid tumors with substantial unmet medical needs, has revealed its intention to broaden its current Phase 1 clinical study. This expansion will encompass two combination cohorts assessing EO-3021 for the therapy of advanced gastric or gastroesophageal junction cancer.
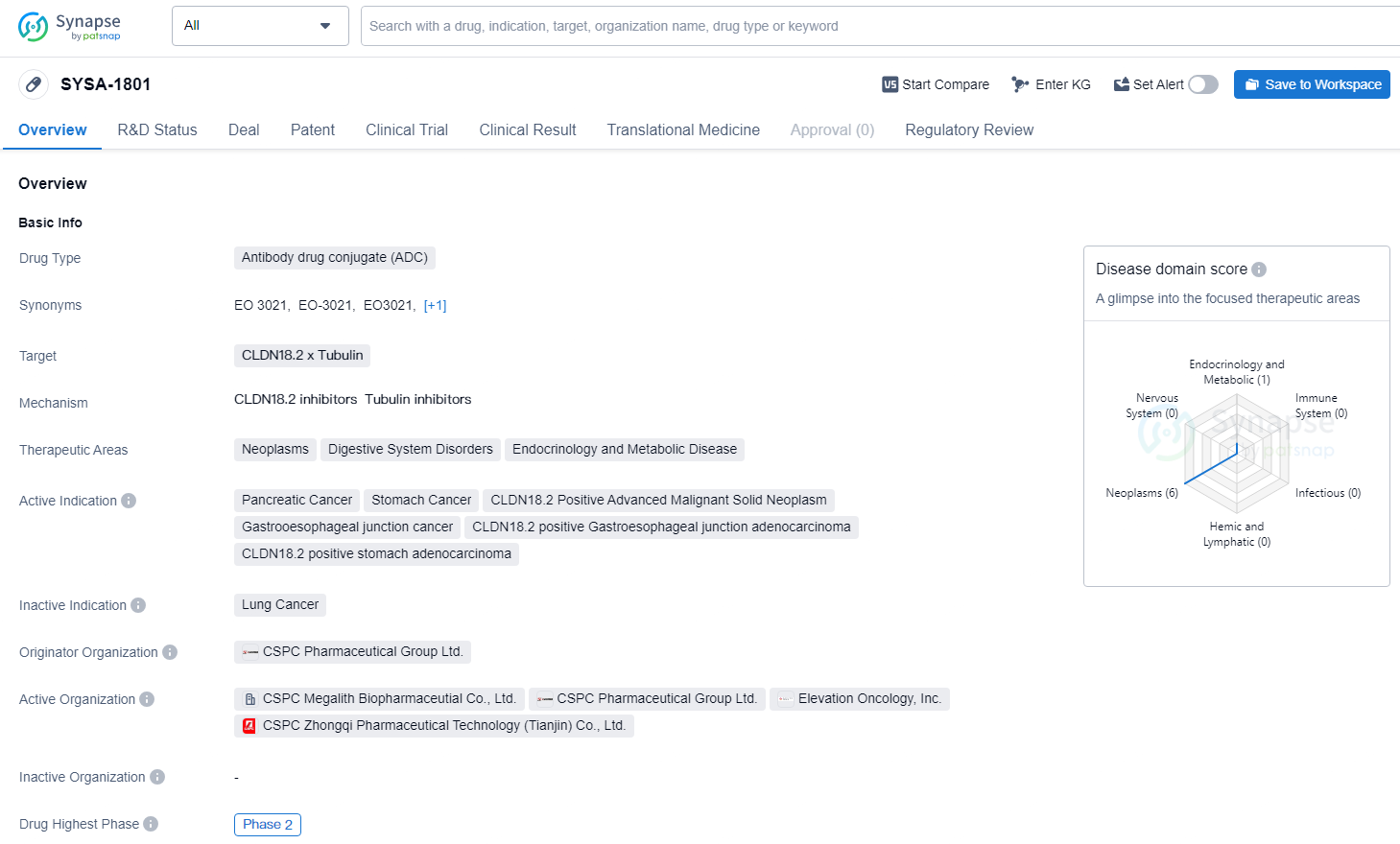
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
After finalizing clinical supply agreements with Eli Lilly and Company and GSK, Elevation Oncology plans to assess EO-3021 in conjunction with the VEGFR2 inhibitor ramucirumab for second-line patients and with the PD-1 inhibitor dostarlimab for front-line patients. The company aims to commence the combination dosing part of the Phase 1 trial by the end of 2024.
"EO-3021 could revolutionize the treatment landscape for tumors expressing Claudin 18.2, which includes most gastric and gastroesophageal junction adenocarcinomas," stated Valerie Malyvanh Jansen, M.D., Ph.D., Chief Medical Officer at Elevation Oncology. She continued, "We are eager to investigate EO-3021 alongside the VEGFR2 inhibitor ramucirumab and the PD-1 inhibitor dostarlimab in both second- and first-line patient settings. Concurrently, we are progressing our Phase 1 trial for EO-3021 monotherapy, with initial safety and efficacy data expected by mid-third quarter."
EO-3021 is an advanced, potentially superior antibody-drug conjugate that targets Claudin 18.2. It employs site-specific conjugation at glutamine to enhance linker-payload stability and reduce the chances of releasing free monomethyl auristatin E compared to traditional cysteine-based linkage methods. Preclinical studies and Phase 1 trials conducted by Elevation Oncology’s partner demonstrated a favorable safety profile with minimal MMAE-related toxicities.
EO-3021 combined with ramucirumab for second-line patients: Ramucirumab, a monoclonal antibody targeting VEGFR2, is approved for use with paclitaxel in treating second-line gastric or gastroesophageal junction cancer after progression following fluoropyrimidine- or platinum-containing chemotherapy. Elevation Oncology aims for EO-3021, in combination with ramucirumab, to offer enhanced safety and efficacy compared to the sanctioned combination of ramucirumab and paclitaxel.
EO-3021 combined with dostarlimab for front-line patients: Dostarlimab is authorized as monotherapy or combined with chemotherapy for certain types of dMMR and MSI-H endometrial cancer in the US, and also has accelerated approval for specific dMMR solid tumors. Elevation Oncology aims to achieve synergistic benefits by combining EO-3021 with an immune checkpoint inhibitor, potentially providing better patient outcomes compared to current frontline immunotherapy treatments for gastric or gastroesophageal junction cancer.
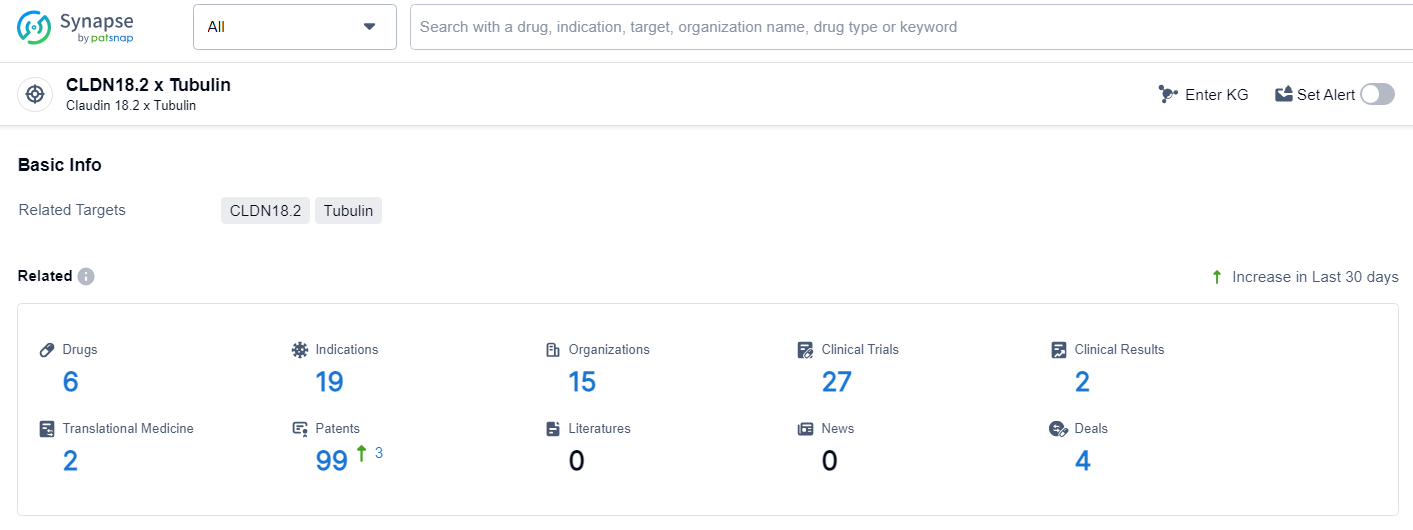
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 3, 2024, there are 6 investigational drugs for the CLDN18.2 and Tubulin target, including 19 indications, 15 R&D institutions involved, with related clinical trials reaching 27, and as many as 99 patents.
EO-3021 is a differentiated, clinical-stage antibody drug conjugate with best-in-class potential comprised of an immunoglobulin G1 (IgG1) monoclonal antibody that targets Claudin 18.2. EO-3021 has reached Phase 1/2 in its global development, and its potential to address unmet medical needs in the specified therapeutic areas makes it a promising candidate for further clinical development and potential commercialization.