Is Fenfluramine approved by the FDA?
Yes, fenfluramine (brand name: Fintepla) is FDA approved. The U.S. Food and Drug Administration (FDA) approved fenfluramine on June 25, 2020, for the treatment of seizures associated with Dravet syndrome in patients two years of age and older.
What is Fenfluramine?
Fenfluramine is an oral medication used to treat seizures caused by Dravet syndrome and Lennox-Gastaut syndrome in patients who are at least 2 years old. These conditions are severe forms of epilepsy that begin in infancy or early childhood and are characterized by frequent, prolonged, and often treatment-resistant seizures.
How Does Fenfluramine Work?
Fenfluramine is classified under drug classes such as anorexiants, CNS stimulants, and miscellaneous anticonvulsants. It works by affecting serotonin levels in the brain, which can help reduce the frequency and severity of seizures.
Usage and Administration
Fenfluramine is available in oral solution form with a concentration of 2.2 mg/mL. The dosage and administration of fenfluramine must be carefully managed by a healthcare provider due to the risk of serious side effects.
Dosage:
- The specific dosage depends on the patient's weight and the severity of the condition.
- The medication can be taken with or without food.
- It's essential to measure the liquid medicine carefully using the provided dosing syringe or a medicine dose-measuring device.
Monitoring:
- Regular monitoring of heart function using an electrocardiograph (ECG) is required due to the risk of cardiac side effects.
- Patients should be weighed regularly, as fenfluramine can cause decreased appetite and weight loss, which may affect growth in children.
Precautions and Considerations
Before Treatment:
- Patients should not use fenfluramine if they have used an MAO inhibitor in the past 14 days due to the risk of dangerous drug interactions.
- Inform your doctor if you have a history of heart problems, weight loss, depression, suicidal thoughts, drug or alcohol addiction, liver, or kidney disease.
- Pregnant women should avoid using fenfluramine, and effective birth control should be used during treatment and for a period after the last dose.
Warnings:
- Fenfluramine can cause serious side effects on the heart and lungs, including chest pain, shortness of breath, and swelling in the lower legs.
- The medication may also affect mood and behavior, potentially leading to anxiety, panic attacks, or suicidal thoughts.
Side Effects
Common Side Effects:
- Increased blood pressure
- Drowsiness
- Decreased weight
- Loss of appetite, vomiting, diarrhea, constipation
- Seizures that do not stop
- Feeling weak or tired
- Fever, infections
- Abnormal heart function tests
- Problems with balance, walking, or muscle movement
- Drooling
- Cold symptoms such as stuffy nose, sneezing, sore throat
Serious Side Effects:
- Serious heart and lung issues
- Suicidal thoughts and behavior changes
- Serotonin syndrome symptoms (agitation, hallucinations, fever, sweating, shivering, fast heart rate, muscle stiffness)
Conclusion
Fenfluramine (Fintepla) received FDA approval on June 25, 2020, for treating seizures associated with Dravet syndrome in patients aged 2 years and older. It is a significant treatment option for managing this severe form of epilepsy, though it requires careful monitoring and management due to potential serious side effects. Always consult with a healthcare provider for personalized medical advice and follow all prescribed guidelines for safe use.
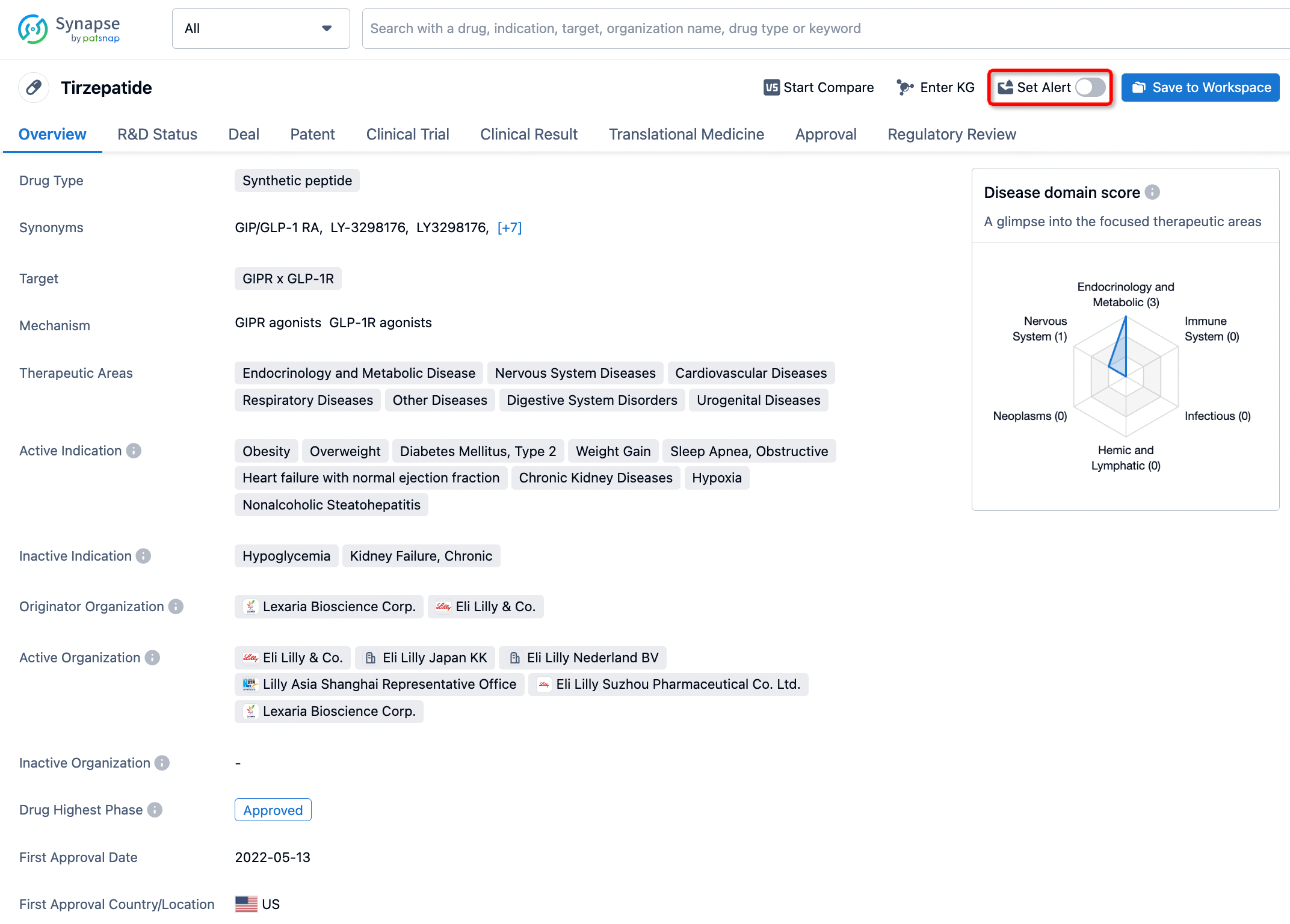
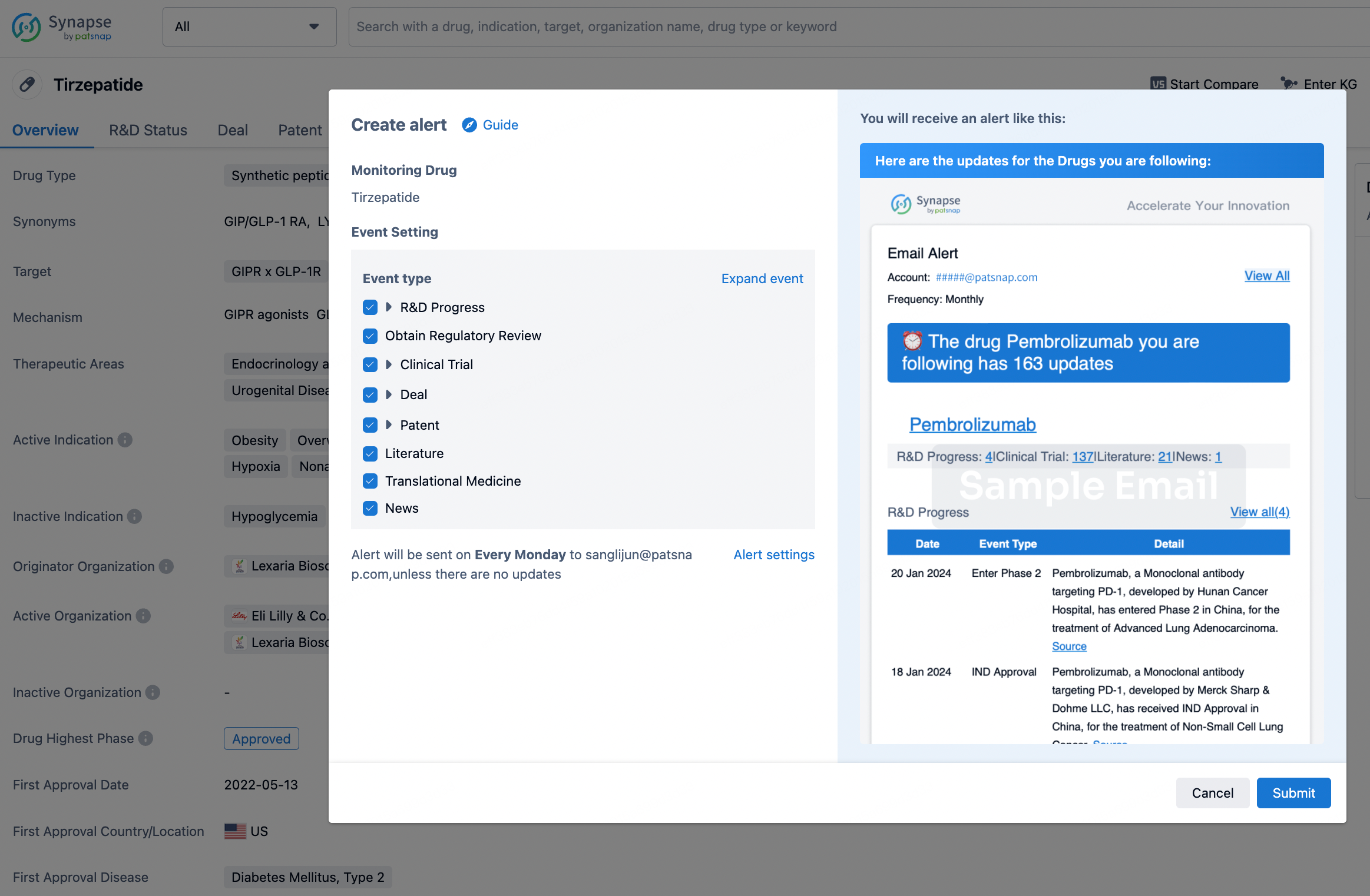
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!