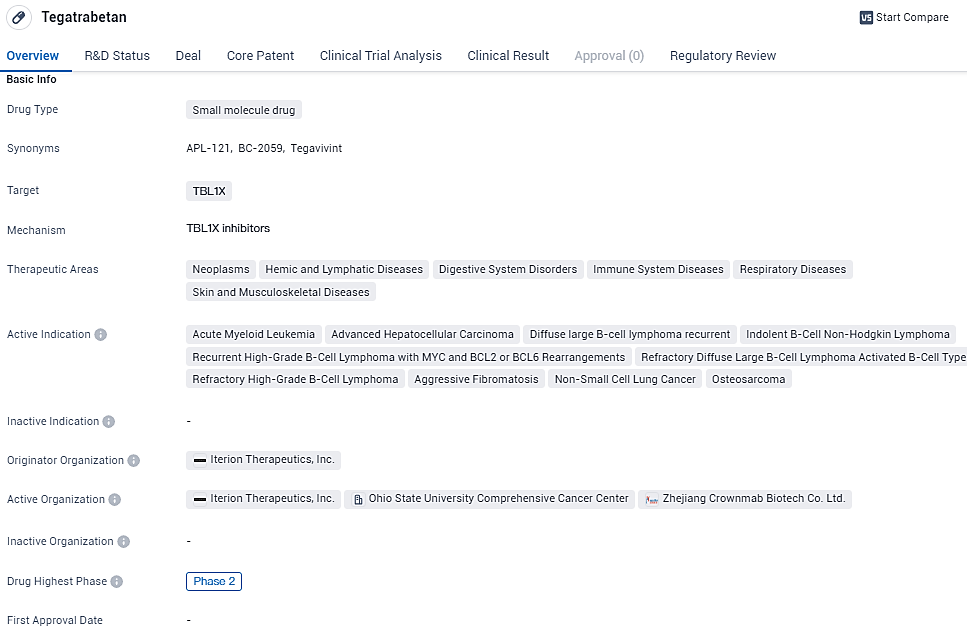
Initial Dosing of Tegavivint in Refractory Advanced Liver Cancer Study Begins
Iterion Therapeutics, a biotech enterprise with a concentration on cancer treatments, is in the process of advancing a new class of small molecule compounds designed to inhibit Transducin beta-like protein 1 (TBL1), which plays a crucial role in the Wnt/beta-catenin signaling cascade. The company has recently publicized that it is progressing with the patient recruitment for its Phase 1b/2a clinical study. This clinical investigation is examining the effects of their prime drug candidate, tegavivint, targeting individuals suffering from progressive hepatocellular carcinoma who have previously undergone and not responded to at least one systemic treatment regimen.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Wnt/beta-catenin signaling corridor is frequently active in multiple cancer variants, significantly in HCC, observing that nearly 50% of affected patients reveal tumor mutations triggering Wnt signals. Regrettably, central components of this signaling route have either been immune to conventional therapeutic compound creation or have been burdened with unrelated detrimental effects.
Diverse and thorough preclinical investigations among a range of cancer types have indicated TBL1 as a critical downstream element vital for the propagation of cancer through Wnt/beta-catenin stimulus. TBL1 adheres to nuclear beta-catenin to fortify the transcriptional complex essential for the initiation of genes leading to cancer. The direct interaction between Tegavivint and TBL1 dislocates beta-catenin, disrupting the complex, instigating the reduction of unattached nuclear beta-catenin, and inhibiting the transcription of genes that drive cancer progression.
Rahul Aras, Ph.D., leading Iterion Therapeutics, articulated, “Focusing on TBL1, Tegavivint exercises effective restraint of nuclear beta-catenin malign influence, circumventing the detrimental effects noted with compounds targeting higher stages of the Wnt-pathway." He asserted the significance of Tegavivint's advancement for HCC patients, a group presently facing a scarcity of treatment alternatives, given the high incidence of Wnt-stimulated tumors.
Casey Cunningham, M.D., Chief Medical Officer at Iterion Therapeutics, highlighted, "The demand is urgent for innovative, targeted treatments to fulfill the vast, unaddressed clinical necessity in HCC cases." He elaborated on Tegavivint’s promising tolerability profile and its capacity to hamper the cancer-promoting activities of beta-catenin in a substantial patient subset.
The active clinical investigation anticipates involving around 35 patients in the dose elevation and refinement phases, focusing on those with inoperable advanced or metastatic HCC who have not responded to prior systemic therapy. The research is poised to assess tolerability, clinical response, and to track pharmacokinetic and pharmacodynamic indicators for determining the Recommended Phase 2 Dose for HCC treatment.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
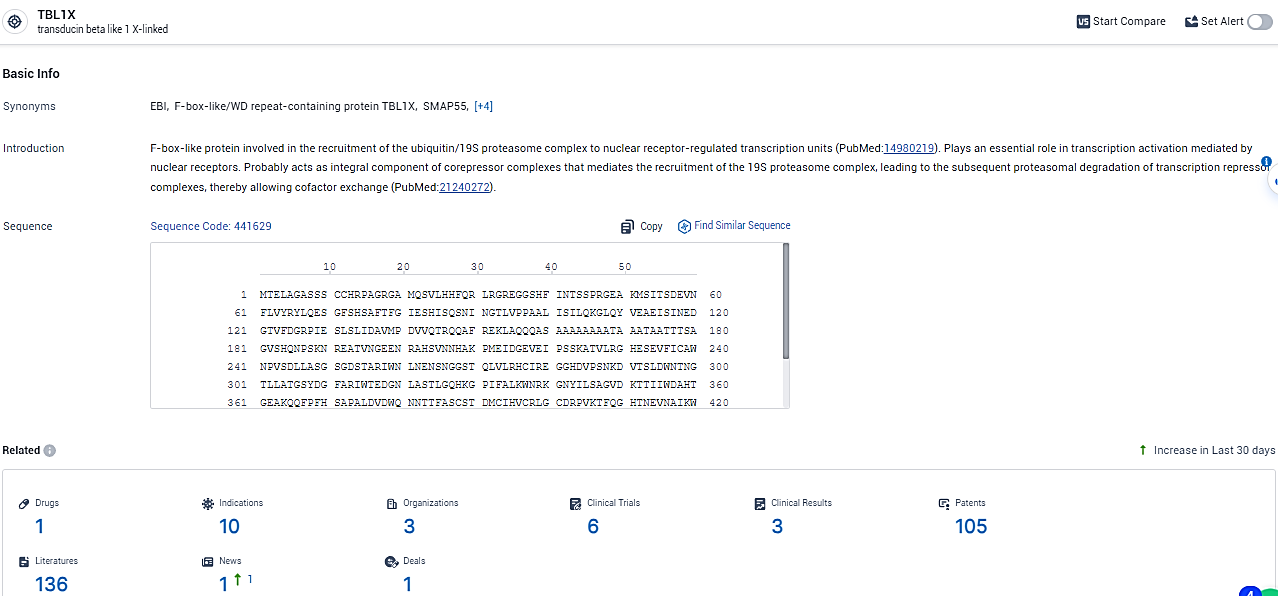
According to the data provided by the Synapse Database, As of February 26, 2024, there are 1 investigational drugs for the TBL1X target, including 10 indications,3 R&D institutions involved, with related clinical trials reaching 6, and as many as 105 patents.
Tegatrabetan shows promise as a potential treatment option for a wide range of diseases and conditions. However, further clinical trials and regulatory approvals are necessary to determine its safety and efficacy. The drug's current phase of development suggests that it has already undergone initial testing and has shown promising results, warranting further investigation.