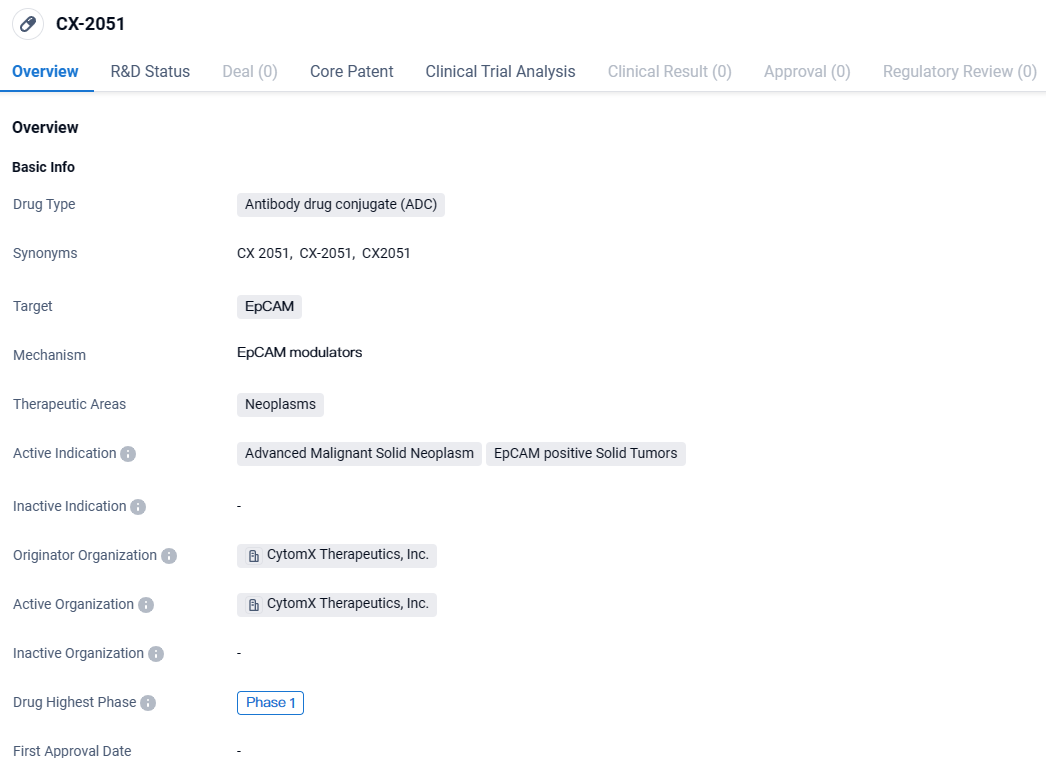
Initial Trial of CytomX's CX-2051, a Targeted ADC for Solid Tumors
CytomX Therapeutics, Inc., a pioneering company specializing in the development of conditionally activated and specifically localized biological treatments, has declared that the initial participant has received a dose within a Phase 1 study. This study is designed to incrementally increase the dosage of CX-2051 and involves individuals who are suffering from advanced-stage solid malignancies.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
CX-2051 is an innovative PROBODY® therapeutic, cloaked and developed as a targeted antibody conjugate against the epithelial cell adhesion molecule (EpCAM), which is frequently overexpressed in various malignancies such as colorectal, gastric, endometrial, and ovarian cancers.
The lethal agent attached to CX-2051 is inspired by the substance camptothecin, renowned for its capacity to inhibit topoisomerase-1, a therapeutic class renowned for its compelling efficacy in combating cancer, and has proven substantial clinical advantages in approved ADC treatments for numerous cancer types.
The initial phase of clinical evaluation for CX-2051, the Phase 1 study, is crafted to rigorously determine the drug's safety profile and its early efficacy against tumors. Results from this early-phase study will provide crucial clinical insights and may shape the decision to progress into broader dosing studies planned potentially for 2025.
"EpCAM holds considerable promise as a focus for ADC strategies, given its abundant expression on several types of solid tumors. With CX-2051, we have harnessed state-of-the-art masking technology and incorporated a robust cytotoxic substance that is particularly well-suited for attacking EpCAM prevalent cancers, such as colorectal tumors," commented Wayne Chu, M.D., the head of clinical affairs at CytomX Therapeutics.
Wayne Chu further remarked, "The launch of the Phase 1 dose escalation trial represents a pivotal step in CytomX's clinical journey, highlighting our commitment to furthering a diverse PROBODY therapeutic portfolio aimed at treating serious diseases that currently lack adequate medical solutions."
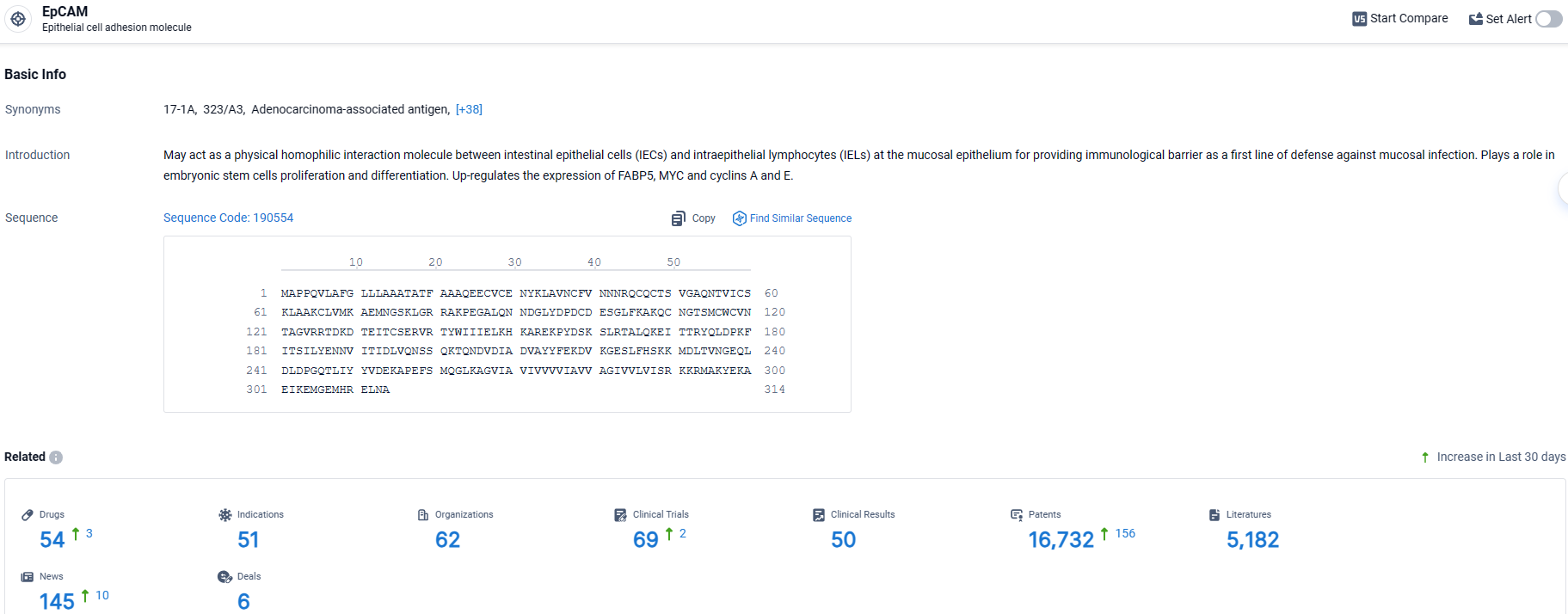
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 10, 2024, there are 54 investigational drugs for the EpCAM target, including 51 indications, 62 R&D institutions involved, with related clinical trials reaching 69, and as many as 16732 patents.
CX-2051 targets EpCAM, a protein overexpressed in certain neoplasms, and is indicated for the treatment of advanced malignant solid neoplasms and EpCAM-positive solid tumors. Currently in Phase 1 of clinical development, CX-2051 has the potential to provide a targeted and potentially more effective treatment option for patients with these types of cancers.