Innate Pharma's Lacutamab Clinical Program resumes with FDA's approval after lifting previous partial hold
Innate Pharma SA has made public that the United States' Food and Drug Administration (FDA) has removed the partial hold previously enforced on the Investigational New Drug application for lacutamab. This announcement, made on October 5, indicated that FDA had partially suspended the lacutamab IND in response to a mortality that occurred in the context of the TELLOMAK trial. Initially, the death was associated with complications arising from hemophagocytic lymphohistiocytosis, which is an uncommon blood-related condition and impacted a patient suffering from Sézary syndrome.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The authorization from the FDA to remove the clinical hold in part can be attributed to an evaluation of a mortality incident by the FDA, which Innate and an autonomous advisory group of specialists concluded was due to the rapid worsening of the condition, not associated with the administration of lacutamab.
"In close collaboration with the FDA, we have meticulously worked through the resolution process of the partial clinical hold placed on the lacutamab Investigational New Drug application, which necessitated a comprehensive examination of the fatality attributed to an advanced stage of the illness," stated Dr. Quaratino, the Chief Medical Officer at Innate Pharma.
Dr. Quaratino also mentioned, "Post the positive disclosures on Sézary syndrome at the latest ASH Annual Meeting 2023, the lacutamab initiative is progressing according to our strategic schedule. We eagerly anticipate the occasion to present comprehensive results for Mycosis Fungoides."
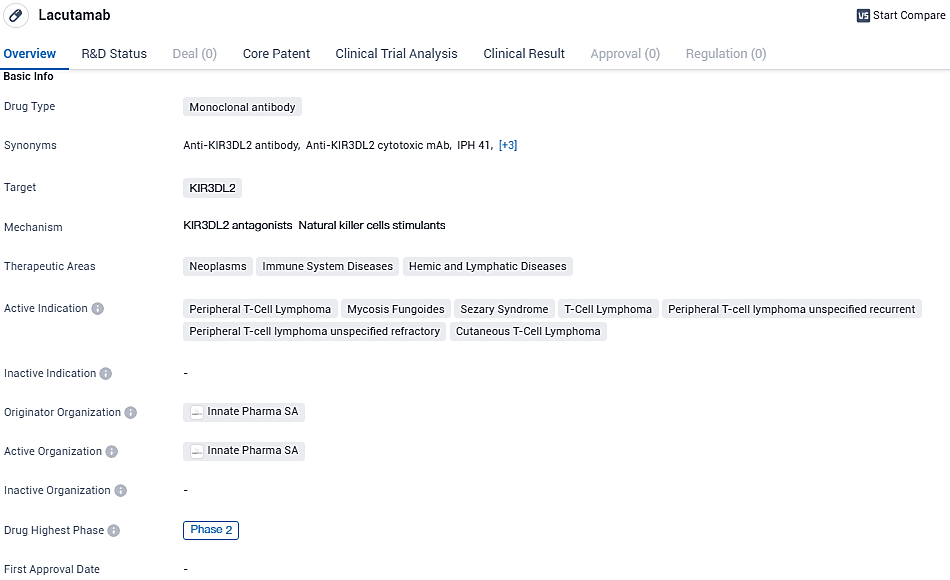
Lacutamab stands out as a novel therapeutic agent classified as an anti-KIR3DL2 humanized antibody with the capability to induce cytotoxicity. It's being investigated clinically as a potential therapy option for certain lymphomas, specifically cutaneous T-cell lymphoma—a disease recognized as orphan due to its rarity—and peripheral T cell lymphoma. These uncommon forms of cutaneous lymphoma, originating in T cells, typically carry a dire outlook and have limited treatment alternatives that are both effective and low-risk, especially at later stages.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
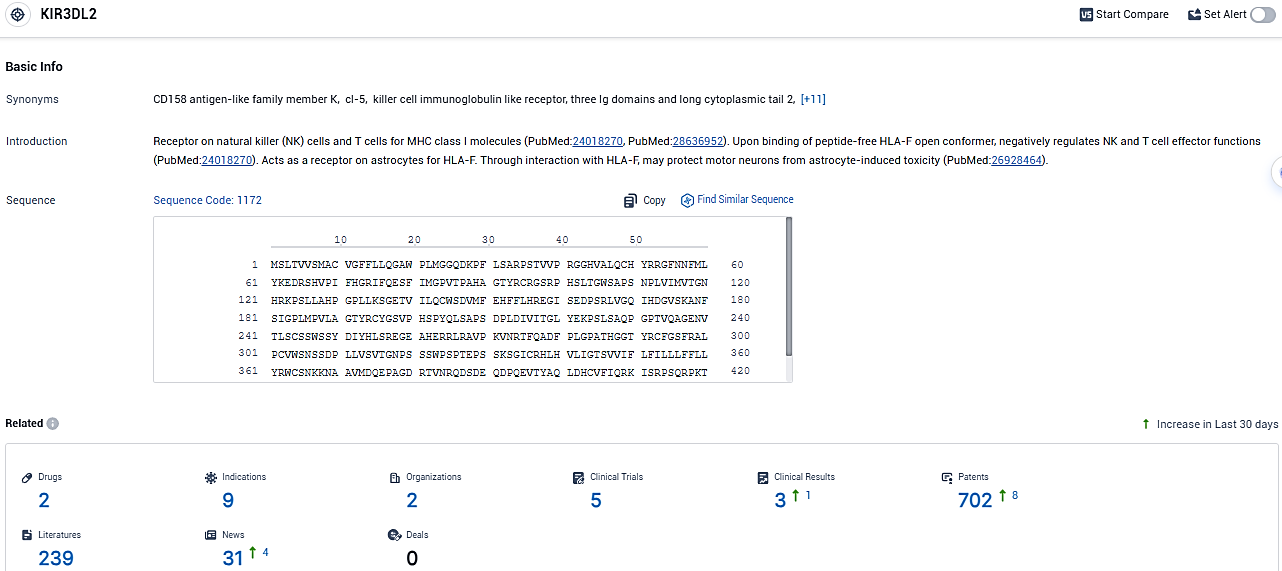
According to the data provided by the Synapse Database, As of January 10, 2024, there are 2 investigational drugs for the KIR3DL2 target, including 9 indications, 2 R&D institutions involved, with related clinical trials reaching 5, and as many as 702 patents.
Lacutamab is granted European Medicines Agency PRIME designation and US Food and Drug Administration granted Fast Track designation for the treatment of patients with relapsed or refractory Sézary syndrome who have received at least two prior systemic therapies. Lacutamab is granted orphan drug status in the European Union and in the United States for the treatment of CTCL.