Innovative Applications of RNA Interference Technology in Drug Development
The development and application of siRNA drugs mark a revolution in the field of gene therapy, as they cleverly utilize the cellular RNA interference (RNAi) pathway to achieve precise regulation of disease-related gene expression. This process begins with the design and synthesis of double-stranded siRNA molecules, which specifically recognize and bind to target mRNA molecules through base pairing principles. This specific binding of siRNA molecules activates the RNA-induced silencing complex (RISC), which unwinds the siRNA duplex and uses one strand as a guide to precisely cleave the complementary mRNA, thereby preventing its translation into functional proteins and achieving gene silencing.
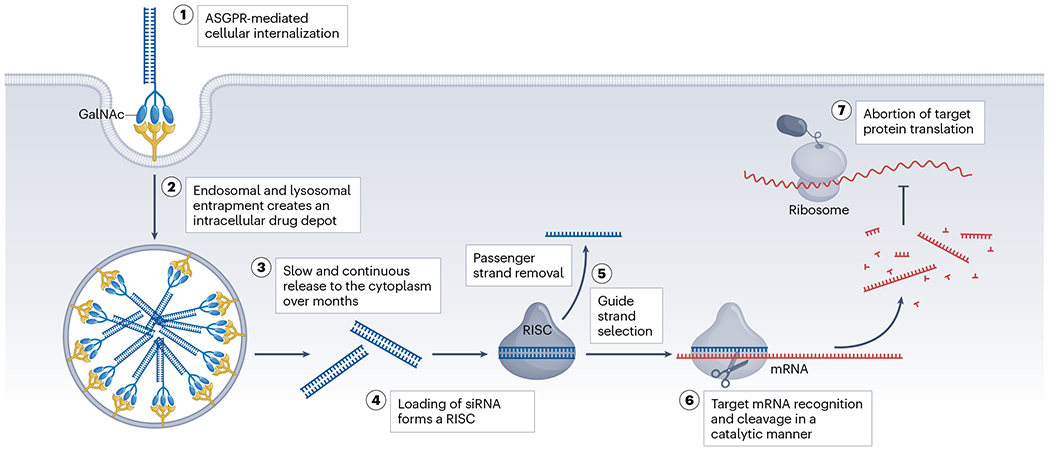
The application prospects of this gene silencing technology are broad, particularly in the fields of hereditary diseases, viral infections, and cancer treatment. For example, Alnylam Pharmaceuticals developed the siRNA drug Onpattro, which targets transthyretin mRNA, reducing the accumulation of abnormal amyloid proteins and providing a new treatment option for patients with hereditary transthyretin-mediated amyloidosis. Givlaari and Oxlumo, respectively, lower the expression of specific enzymes in the liver to treat acute intermittent porphyria and primary hyperoxaluria. Leqvio, by reducing the expression of PCSK9 protein, offers an innovative lipid-lowering therapy for patients with hypercholesterolemia. Amvuttra and Reglamento target different hereditary metabolic disorders, using siRNA technology to regulate key metabolic pathways and improve clinical symptoms.
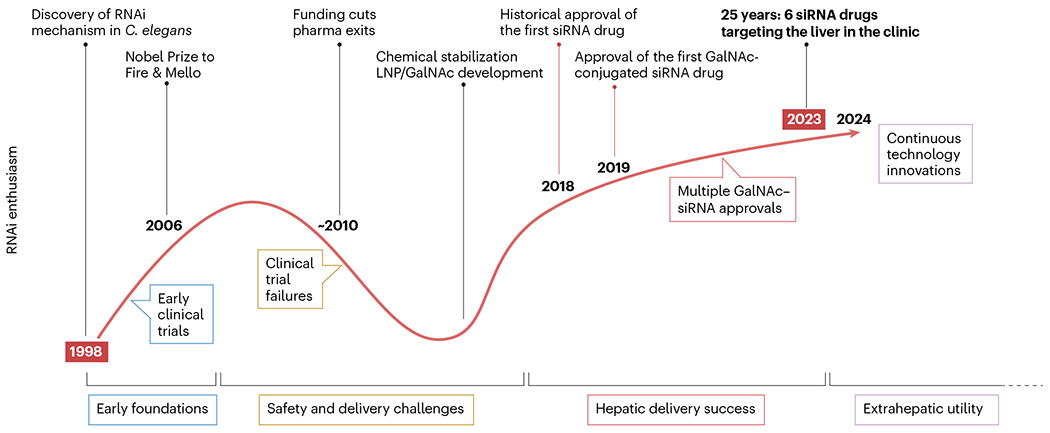
A deep understanding of the sequence characteristics and design methods of approved siRNA drugs is crucial for advancing the development of new siRNA therapies. By analyzing the sequences of these drugs, researchers can identify sequence patterns related to efficacy, specificity, and safety. Patsnap Bio provides a comprehensive resource that enables researchers to access and compare the sequence information of different siRNA drugs, accelerating the drug discovery and development process. Through this information, researchers can optimize the design of siRNA, enhancing its clinical potential and offering new therapeutic options for treating various diseases.
siRNA Drug Design – Off-Target Effects
The key to siRNA drug design lies in achieving high target specificity to avoid off-target effects and potential non-specific gene silencing. To enhance siRNA specificity, multiple factors need to be considered during the design process, including the degree of mismatch with potential off-target sites, the complementarity of the seed region, and sequence characteristics that may affect chemical, manufacturing, and control (CMC) properties.
In genome-wide RNA screening, seed region-based off-target effects pose a significant challenge. The 2-8 nucleotide region at the 5' end of the siRNA (the seed region) is crucial for guiding the RNA-induced silencing complex (RISC) to recognize and bind to the target mRNA. If the seed region of the siRNA has sufficient complementarity with the 3' untranslated region (UTR) of non-target mRNA, it may lead to non-specific gene silencing, known as off-target effects.

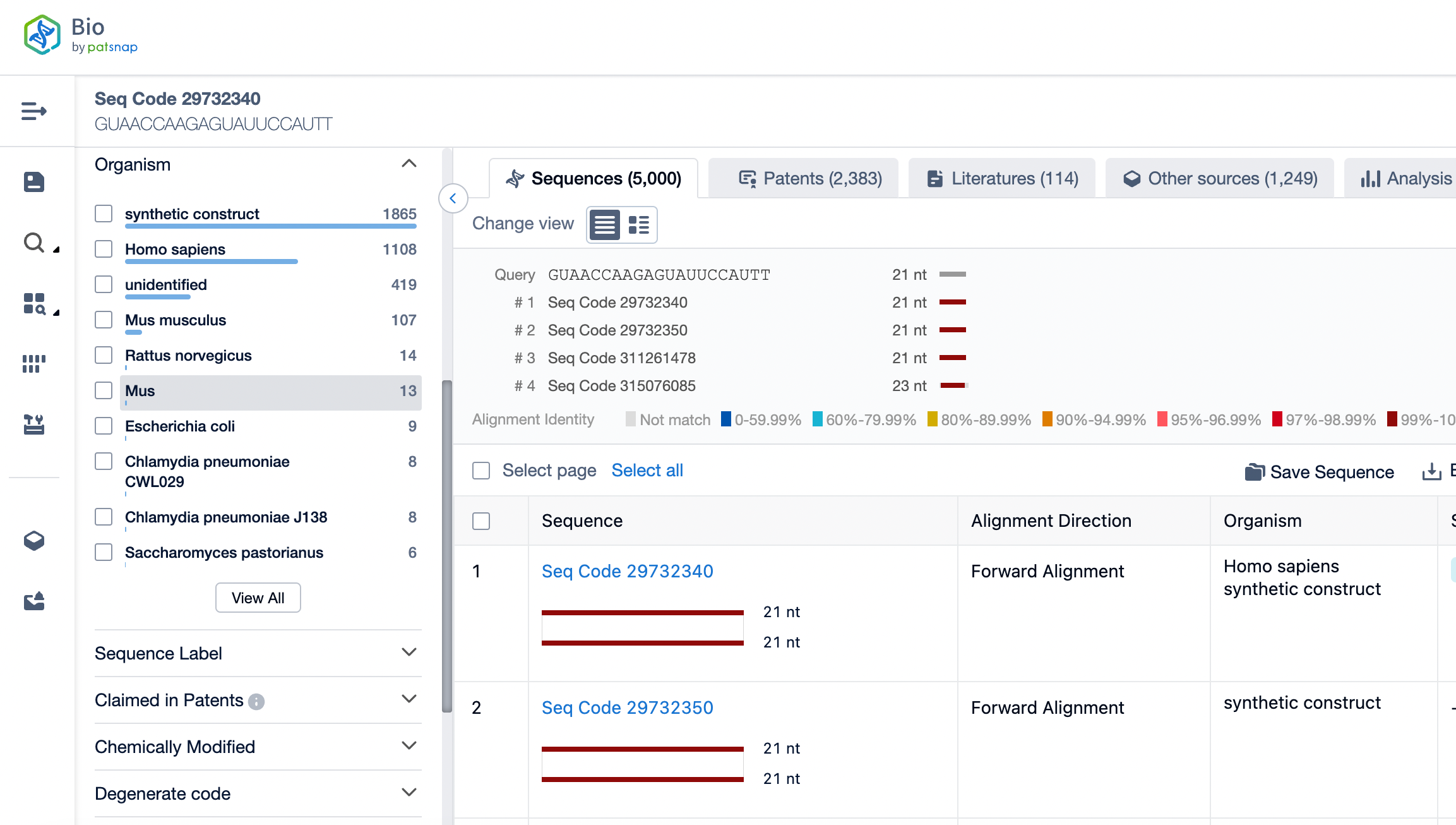
siRNA Drug Design – Sequence Selection
We use Patsnap Bio to analyze the sequence characteristics of Onpattro (Patisiran), the world’s first approved siRNA drug. The siRNA molecule in Onpattro consists of two complementary strands, with the antisense and sense strands each containing 21 bases. These strands form a stable double-stranded structure through precise base pairing. This structural design grants the siRNA molecule high specificity, allowing it to specifically bind to the mutant transthyretin (TTR) mRNA associated with hereditary transthyretin-mediated amyloidosis (hATTR). This guides the RNA-induced silencing complex (RISC) to the target mRNA, triggering its cleavage and degradation, thereby achieving precise regulation of disease-related gene expression.
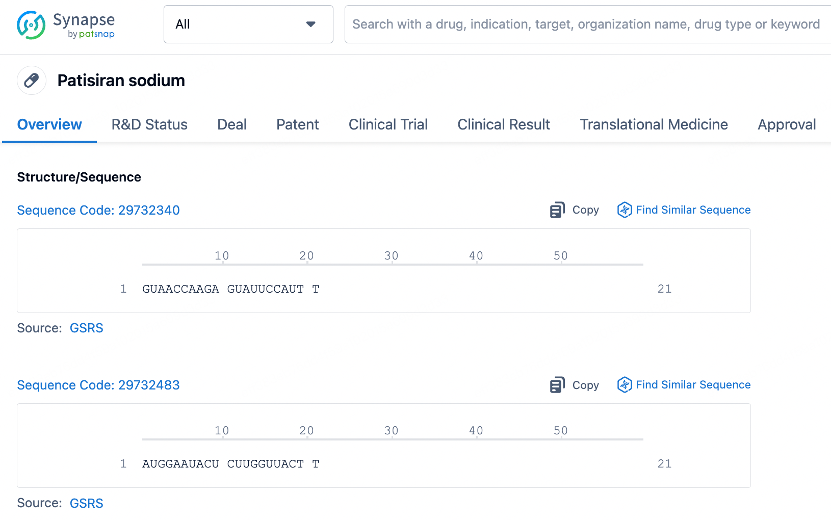
In the mechanism of action of siRNA drugs, the RISC processes the double-stranded siRNA molecule, unwinds it, and selects one of the strands as the guide strand, while the other strand is degraded. The guide strand binds to the complementary sequence of the target mRNA, triggering the RNA interference (RNAi) pathway, which leads to the cleavage and degradation of the target mRNA. Which strand is chosen as the guide strand depends on its sequence characteristics, especially the thermodynamic stability at both ends. The RISC complex tends to select the strand with lower thermodynamic stability as the guide strand, as this strand is more easily unwound from the siRNA duplex. Patsnap Bio allows us to analyze the thermodynamic properties and other biological characteristics of the siRNA sequence to predict and validate guide strand selection.
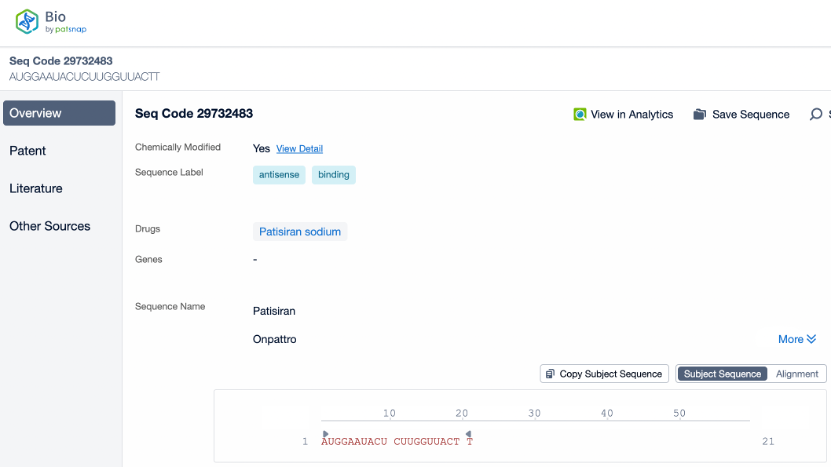
The other strand, known as the passenger strand, is usually degraded after the siRNA is processed by the RISC. However, it is noteworthy that in certain cases, the passenger strand may also have biological functions. It can influence the loading process of RISC or affect the stability of siRNA in certain cell types. Using Patsnap Bio, we gained detailed insights into Onpattro’s sequence information and deepened our understanding of the role of the passenger strand in drug design and function.
siRNA Drug Design – Cross-Species siRNA Design
Using Patsnap Bio for alignment, we can identify conserved sequence regions across species, which helps in determining the preferred targets for siRNA design. Ensuring that siRNA shares the same sequence homology between the human target and animal models in preclinical studies improves both the accuracy and relevance of the research. Moreover, for diseases that are difficult to model in humans, specific animal models may provide environments that better mimic human pathological conditions. In these models, cross-species siRNA design not only aids in establishing more accurate disease models but also serves as a tool for testing the efficacy of siRNA drugs. For instance, in the preclinical studies of Onpattro, crab-eating macaques and transgenic mice were used as model animals to determine the safety and efficacy of the siRNA drug.
Summary
The development of siRNA drugs leverages the natural mechanism of RNA interference (RNAi). By designing siRNA molecules with precision, researchers can specifically target and silence disease-related genes, holding revolutionary potential for treating genetic diseases, viral infections, and cancer. The success of Alnylam Pharmaceuticals’ siRNA drugs has demonstrated the feasibility of using the RNAi pathway for therapeutic purposes, underscoring the importance of understanding siRNA sequence characteristics and design methods. With Patsnap Bio, researchers can analyze the sequence properties of siRNAs, predict guide strand selection in vivo, and assess their cross-species activity, providing scientific support for the design and optimization of siRNA drugs.
Cross-species siRNA design is a critical strategy in drug development, as it not only enhances the accuracy of preclinical studies but also supports disease model development and drug efficacy testing. By conducting comparative analysis using Patsnap Bio, researchers can identify conserved sequence regions across species, guiding the selection of preferred targets for siRNA design. This approach facilitates validation of siRNA activity in various biological models, accelerating the transition from laboratory research to clinical application. Furthermore, cross-species siRNA design aids in understanding the biological functions of siRNA in different species, including its role in the RISC complex and the potential functions of the passenger strand. These insights are not only crucial for current drug development but also lay the foundation for future innovations in siRNA technology.
Better answers for better bio-innovations!
Validate novelty, eliminate risk, and innovate with confidence using the world’s largest sequence database curated from millions of patent and non-patent sources.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.
With best-in-class coverage of protein and nucleic acid sequences combined with state-of- the-art search algorithms, you’ll spend less time searching and more time bringing your bio-innovations to market.
Reference
Tang, Q. & Khvorova, A. RNAi-based drug design: considerations and future directions. Nat Rev Drug Discov 23, 341-364 (2024). https://doi.org:10.1038/s41573-024-00912-9




