Senhwa Biosciences Files IND with FDA for Pidnarulex Study in Advanced Solid Tumors
Senhwa Biosciences, Inc. (TPEx: 6492), a biopharmaceutical company dedicated to developing innovative therapies for cancer, rare diseases, and infectious diseases, announced that the National Cancer Institute (NCI) has submitted an Investigational New Drug (IND) application for Pidnarulex to the U.S. FDA. Pidnarulex (CX-5461), an investigational drug created by Senhwa, has been chosen as a candidate in the NCI's NExT (NCI Experimental Therapeutics) program. This initiative is funded by the Division of Cancer Treatment and Diagnosis (DCTD) of the NCI, which is a component of the U.S. National Institutes of Health (NIH), and will extend over a five-year timeframe. The drug is set to be employed in a pharmacodynamic (PD) pilot study targeting patients with advanced solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In addition to this monotherapy study, the National Cancer Institute (NCI) is considering future clinical trials to investigate Pidnarulex (CX-5461) in conjunction with other treatments, such as immunotherapy, antibody-drug conjugates (ADCs), and PARP inhibitors (Poly ADP-ribose Polymerase inhibitors, PARPi). If these trials proceed, they will be spearheaded by the NCI, utilizing its medical professionals, network of scientific experts, and regulatory resources—capabilities that many biotech firms would find challenging to achieve on their own. This assistance from the NCI aims to considerably expedite the development and broaden the application of Pidnarulex (CX-5461), with the goal of bringing it to market sooner to benefit patients.
The clinical trial, with the IND application filed by the NCI’s Division of Cancer Treatment and Diagnosis (DCTD), seeks to assess the response of various biomarkers to Pidnarulex (CX-5461) in patients with or without homologous recombination deficiency (HRD).
Pidnarulex (CX-5461), created by Senhwa, is an innovative small-molecule that stabilizes G-quadruplex (G4) structures, often found in the promoters of oncogenes. By halting replication fork progression, Pidnarulex induces DNA damage, leading to the death of cancer cells. This mechanism allows Pidnarulex to emerge as a promising therapeutic agent for a variety of cancers.
Recently, immunotherapy has emerged as the rapidly growing segment in the oncology drug market, and the advancement of antibody-drug conjugates (ADCs) is undeniably a significant trend in biopharmaceuticals. Leading pharmaceutical corporations, such as Pfizer, AbbVie, AstraZeneca, and Merck, have invested billions in acquiring or licensing relevant technologies. According to a recent report by the market research firm Evaluate, the ADC market is projected to reach $30 billion by 2028, while the global cancer immunotherapy market is expected to exceed $224 billion by 2030.
Given that immunotherapy is effective in approximately 20% to 25% of patients, the combination of immunotherapy with targeted drugs is becoming increasingly vital in cancer treatment. Combination therapies can target multiple pathways within the complex tumor microenvironment, potentially enhancing immunotherapy's effectiveness, making it a key research focus for major pharmaceutical companies. Consequently, Senhwa is optimistic and confident in the NCI’s plans to advance clinical trials of Pidnarulex (CX-5461) in combination with immunotherapy and ADCs.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
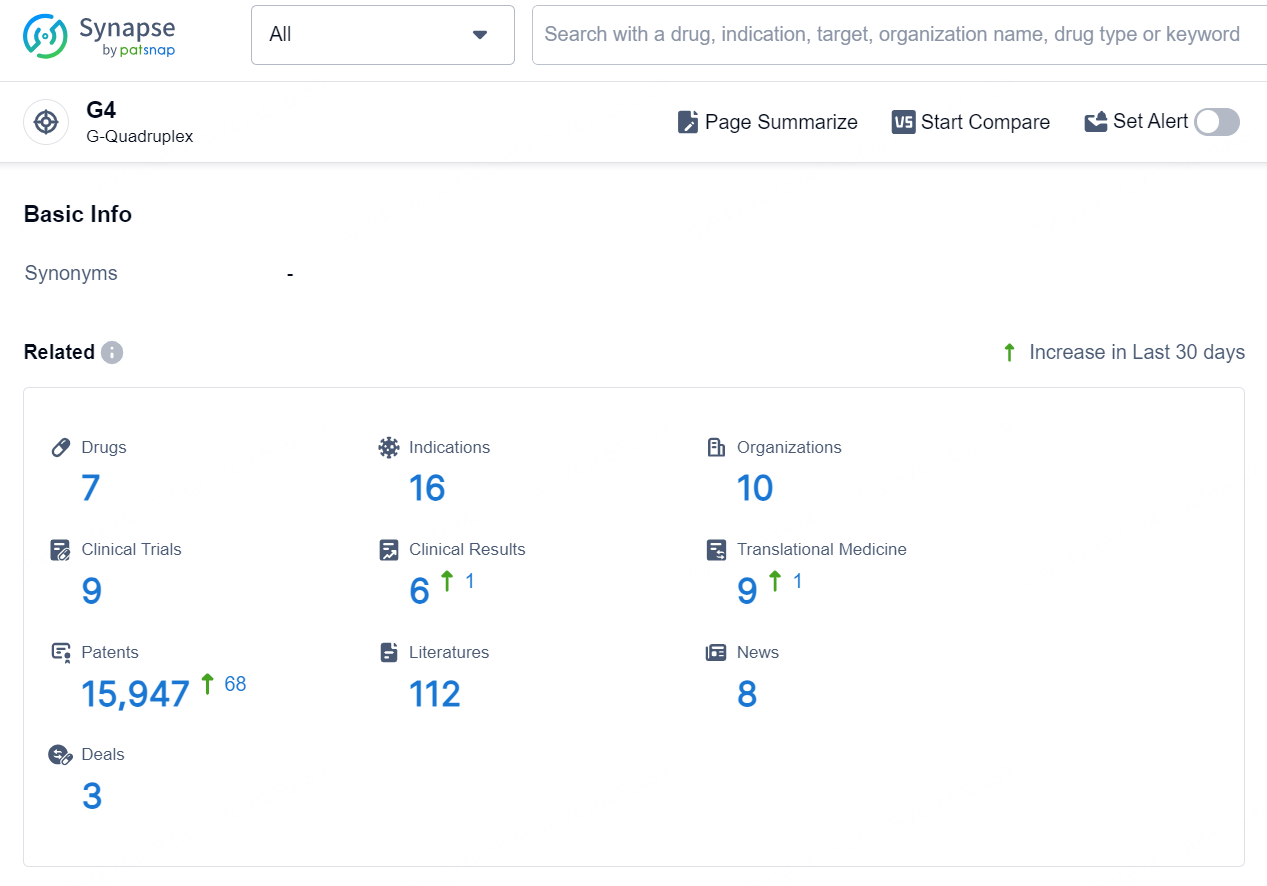
According to the data provided by the Synapse Database, As of September 18, 2024, there are 3 investigational drugs for the G4 target, including 5 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 434 patents.
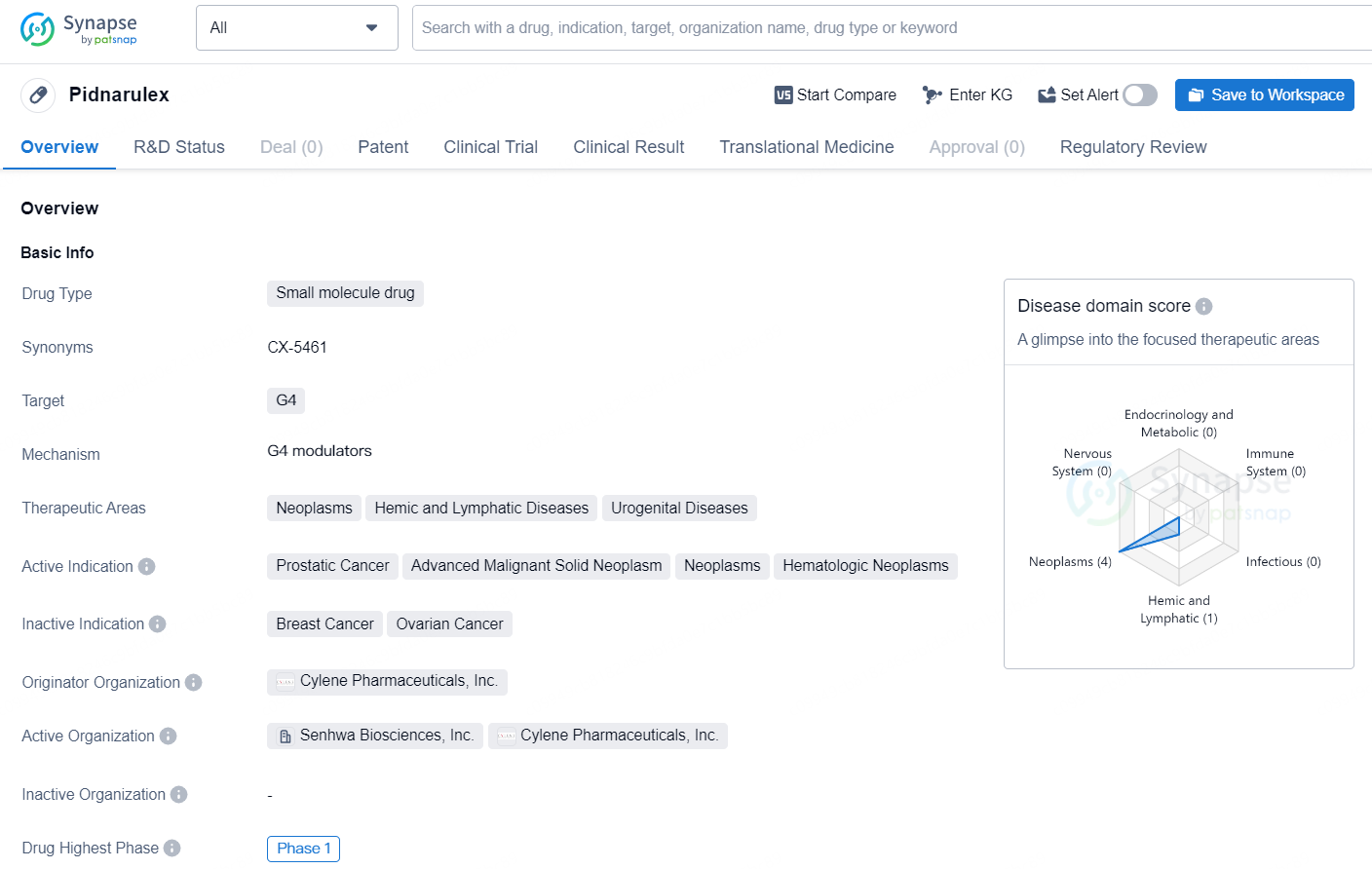
Pidnarulex is a small molecule drug developed by Cylene Pharmaceuticals, Inc. The drug targets G4 and is currently in the highest phase of development globally, which is Phase 1. Pidnarulex is primarily focused on therapeutic areas such as neoplasms, hemic and lymphatic diseases, and urogenital diseases. The active indication for Pidnarulex includes prostatic cancer, advanced malignant solid neoplasm, neoplasms, and hematologic neoplasms.