Nature: Identification of Allosteric Modulators Targeting Opioid Receptors through DNA-Encoded Small Molecule Library Screening
Recently, Professor Brian Kobilka from Stanford University, along with his collaborators, published a research paper titled "A µ-opioid receptor modulator that works cooperatively with naloxone" in the journal Nature. The paper discusses the identification of a novel negative allosteric modulator of the µ-opioid receptor (µOR) through screening with a DNA-encoded small molecule library. This modulator can work synergistically with the antagonist naloxone to counteract the effects of opioid overdose.

The researchers explored the modulation of receptor activity by binding molecules at allosteric sites on the receptor, rather than further exploring agonism at the traditional orthosteric sites. Allosteric modulators may act more specifically on the µOR (as opposed to its κ-opioid receptors (κOR) and δ-opioid receptors (δOR)).
Opioid receptors play a crucial role in pain treatment, and their activation can inhibit the transmission of pain signals, significantly alleviating various acute and chronic pain conditions, such as postoperative pain and cancer pain. Specific agonists for the μ (mu), δ (delta), and κ (kappa) receptor subtypes, such as morphine, oxycodone, and fentanyl, bind selectively to their respective receptors, triggering intracellular signaling pathways that reduce neuronal excitability, thereby producing potent analgesic effects.
However, opioid-mediated analgesia also comes with a series of challenges. Long-term use can lead to tolerance, necessitating increasing doses to maintain efficacy, and may also result in physical and psychological dependence, increasing the risk of addiction. Some opioid analgesics have diverse adverse reactions, such as respiratory depression, constipation, nausea, vomiting, etc., which can threaten life safety and may lead to withdrawal symptoms after discontinuation, requiring a gradual reduction in dosage or adjunctive medication.
It is reported that in more than 30% of people who died from drug overdose, naloxone was detected. Naloxone is an opioid antagonist and is the most common and effective method for treating opioid overdose. It can be administered via intravenous (IV), intraosseous (IO), intramuscular (IM), intranasal (IN), or subcutaneous (SC) injection. It is used to partially or completely reverse opioid depression caused by natural and synthetic opioids, including respiratory depression. The duration of action of some opioids exceeds that of naloxone, so repeated doses of naloxone may be required.
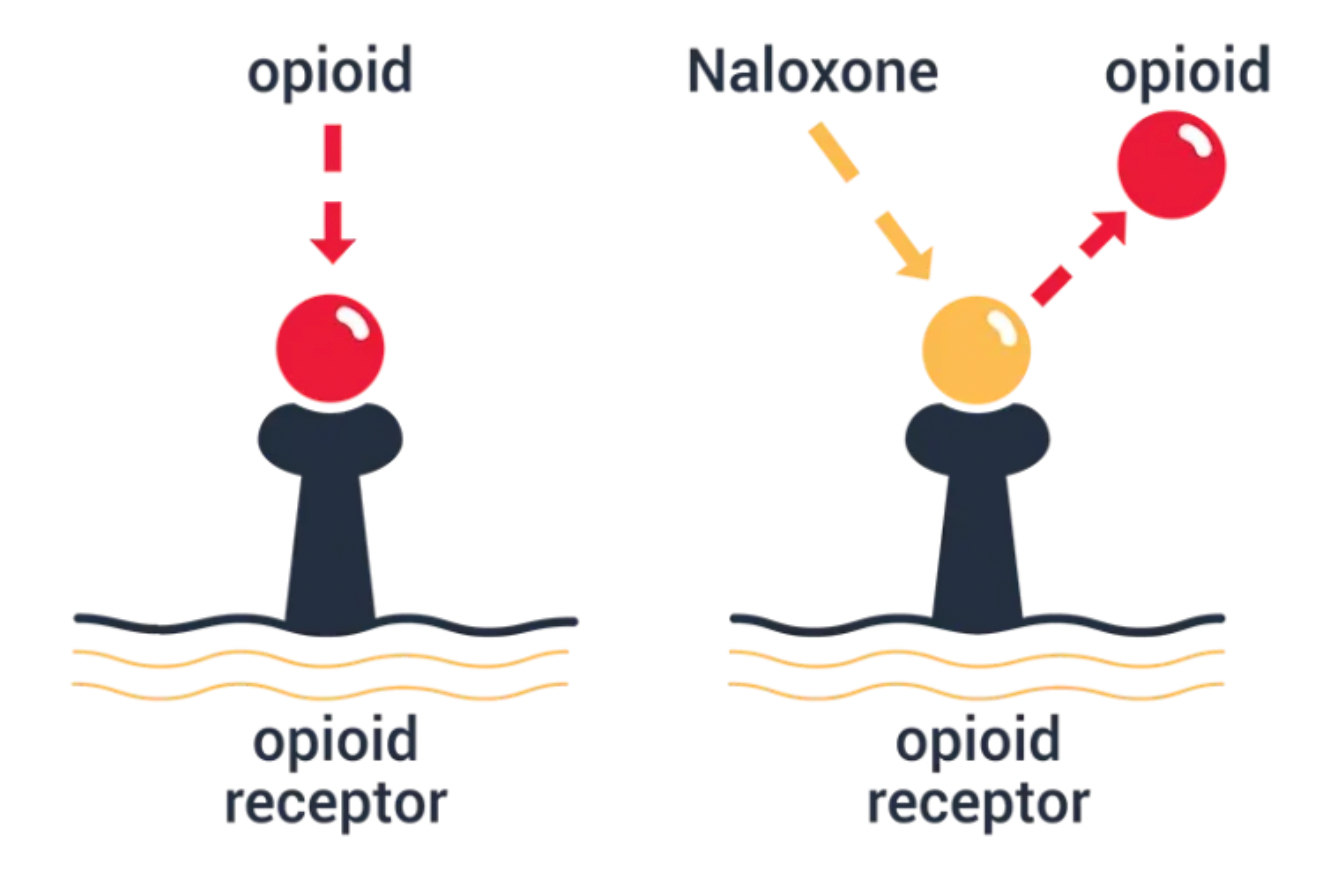
Given the limitations of current opioid drugs (including agonists and antagonists) and the severity of the current opioid epidemic, there is a strong desire to develop novel opioid receptor modulators with different mechanisms of action, which has become a priority for the National Institute on Drug Abuse.
Research Analysis
In order to discover negative allosteric modulators (NAMs) that inhibit receptor activation, researchers utilized a 4.4 billion molecule diverse DNA-encoded compound library (DELs) provided by WuXi Apptec. They combined forward screening (receptor stabilized by antagonists) and reverse screening (receptor stabilized by agonists) to exclude molecules that are not conformation-sensitive or have a specific stabilizing tendency for receptor conformations.
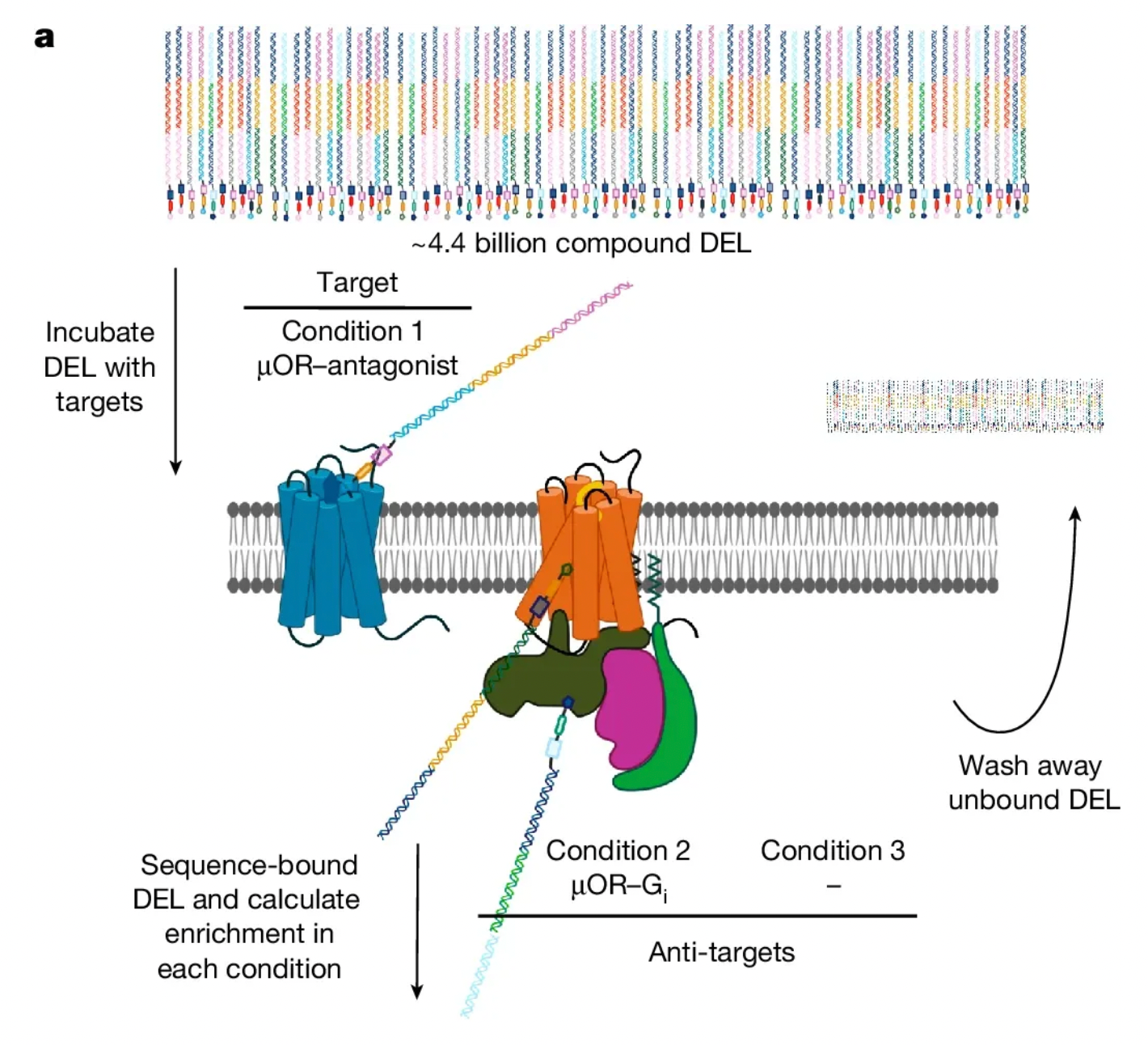
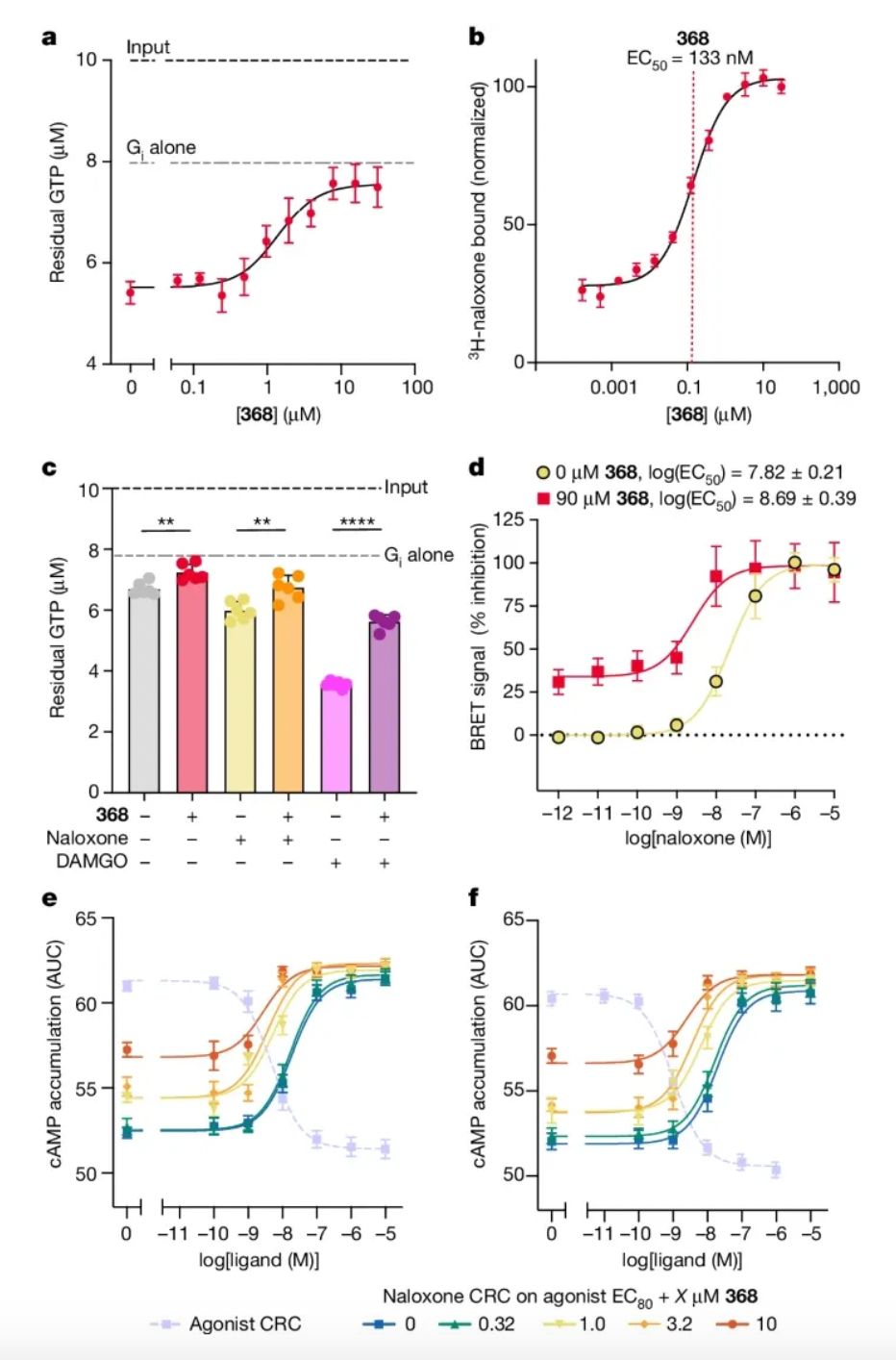
The screening experiment identified a compound named 368, which demonstrated the functionality of a classic negative allosteric modulator in multiple dimensions of biological activity testing. For instance, it showed activity in inhibiting GTP transformation in vitro in the GTP-binding assay; it enhanced the affinity of the antagonist naloxone in isotope-binding assays; and it exhibited the effect of assisting naloxone in antagonizing the efficacy of agonists in cellular level activity tests (such as TRUPATH and cAMP activity assays).
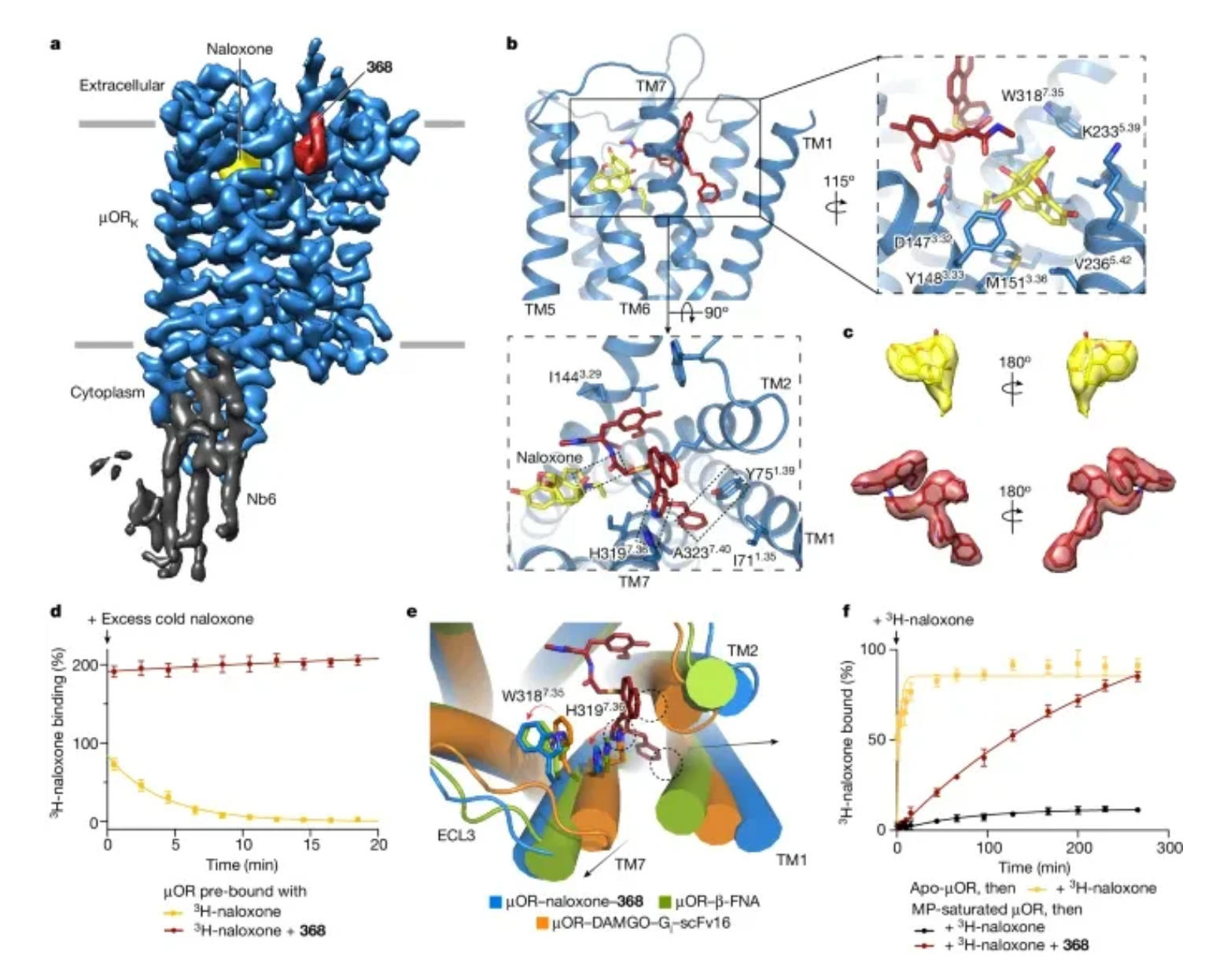
Structural studies using cryo-electron microscopy provided insights into the complex structure of 368 bound with naloxone in the extracellular region of the receptor, studying the recognition mechanism and allosteric modulation mechanism of 368 at the molecular and structural levels. Compound 368 not only stabilizes the recognition conformation of naloxone but also reduces the dissociation rate of naloxone. Molecular dynamics studies also provided data support for the synergistic effects observed in the structure.
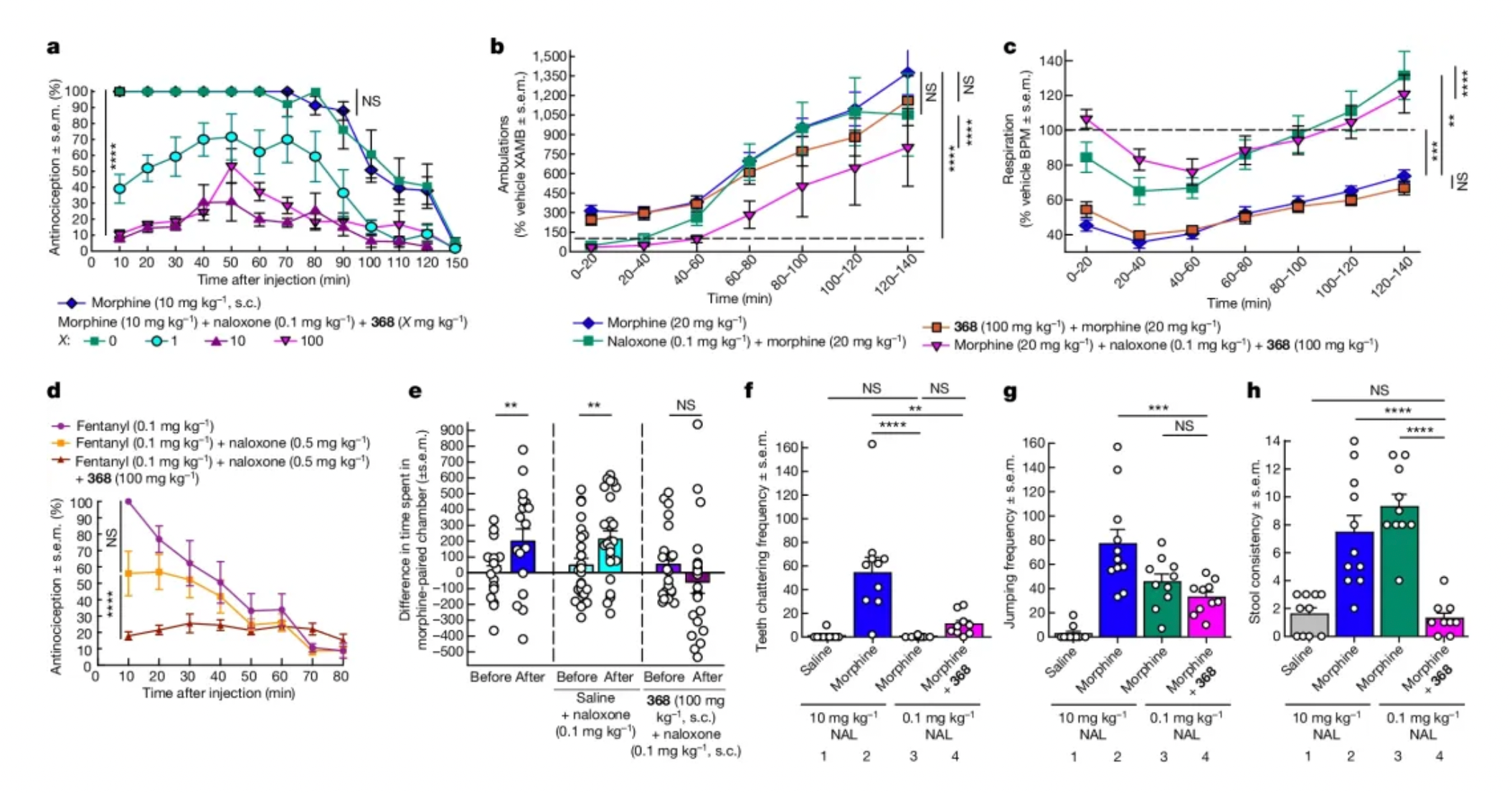
Considering the synergistic and potentiating effects of naloxone (Naloxone) in the in vitro activity tests of 368, researchers have also investigated the functional impact of 368 in a mouse model. The results showed that 368 significantly enhanced the effects of low-dose naloxone in countering hyperactivity and respiratory depression induced by morphine, but did not significantly enhance the withdrawal symptoms caused by low-dose naloxone.
·Compound 368 enhances the effect of low-dose naloxone against morphine: Researchers found that although the low dose of naloxone alone had a weak or insignificant effect on the antinociceptive effect (referring to the ability to relieve pain) induced by fentanyl, when used in combination with 368, it significantly enhanced naloxone's reversal of the antinociceptive effect induced by fentanyl.
·Compound 368 enhances the effect of low-dose naloxone against fentanyl: The results showed that all doses of naloxone had a weak or insignificant effect on the effects of fentanyl without 368. However, regardless of the dose of naloxone, when used in combination with 368, it significantly enhanced naloxone's reversal of the antinociceptive effect induced by fentanyl.
·Researchers also explored whether 368 could enhance the withdrawal symptoms induced by low-dose naloxone: The results showed that low-dose naloxone alone had no effect on the frequency of teeth chattering, and although the combination with 368 increased withdrawal symptoms, it was significantly lower than the response to high-dose naloxone treatment. For other indicators, such as jumping frequency, low-dose naloxone alone induced significant withdrawal symptoms, but there was no significant change when 368 was present. In addition, although both low and high doses of naloxone treatment induced significant diarrhea in morphine-dependent mice, the combination of low-dose naloxone with 368 did not significantly alter the consistency of the feces.
In the study, based on the discovery of compound 368, researchers propose that new drugs may be developed in the future that can more effectively combat the addiction and abuse issues associated with opioid drugs, while reducing side effects such as respiratory depression. By further investigating the SAR relationship between 368 and naloxone (Naloxone), improving the metabolic half-life of 368, its blood-brain barrier permeability, providing the potency of the compound, and the subtype selectivity of the opioid receptor, it may be possible to improve existing treatment strategies for opioid overdose and provide more effective treatment plans. Additionally, utilizing the concept of allosteric modulators, future drug design may consider regulating receptor activity through non-traditional binding sites, thereby developing drugs with new mechanisms of action.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!