Innovent Commences Australian Phase 1 Trial Dosing for Dual-Action Antibody IBI3002
Innovent Biologics, Inc., has declared that the initial volunteer in Australia has received their first dose in the pioneering phase 1 human clinical study of IBI3002. This novel bispecific antibody is distinguished by its unique targeting mechanism aimed at the Interleukin 4 receptor α (IL-4Rα) and thymic stromal lymphopoietin (TSLP) receptors.
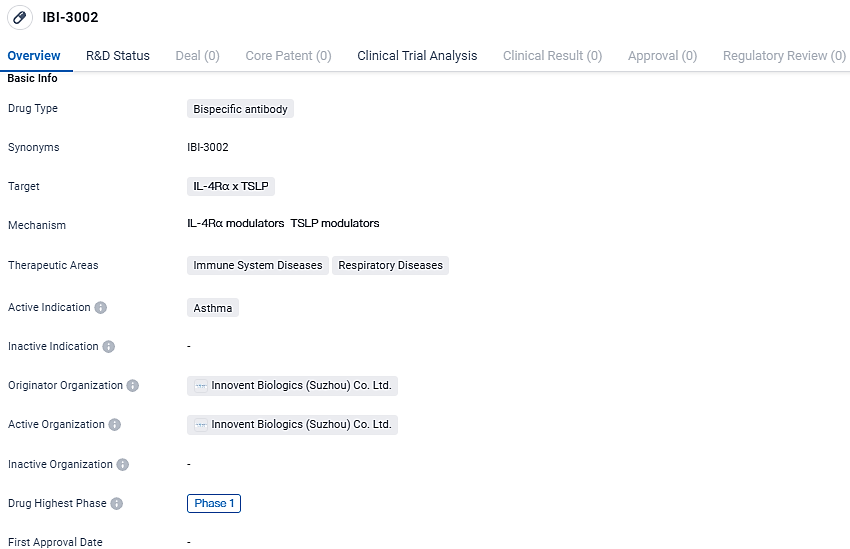
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This initial in-human (FIH) evaluation is conducted as a blind, randomized trial with a placebo control, involving gradual escalation of single doses. The objective is to ascertain the security profile, tolerability levels, pharmacokinetic properties, and pharmacodynamic effects of IBI3002 in both healthy subjects and those suffering from mild-to-moderate asthma. The research will lay the groundwork for the wider international progression of IBI3002's clinical trials.
The therapeutic agent IBI3002 is a human-made, dual-specific antibody created by the company Innovent, aimed at both the IL-4Rα receptor situated on cell surfaces and the clandestine alarmin cytokine known as TSLP. This design is strategic for the management of inflammation-related conditions such as asthma. IL-4 receptors are significant conduits for both IL-4 and IL-13 signals, both pivotal for managing type 2 inflammatory illnesses. The cytokine TSLP, produced by epithelial cells, is instrumental in instigating various asthmatic inflammatory responses, both T2 and beyond.
IBI3002 has proven exceptionally effective in dual-inhibition actions against IL-4Rα and TSLP. Laboratory studies suggest that it offers advantages over other single-target monoclonal antibodies currently available. By confronting IL-4Rα and TSLP simultaneously, IBI3002 is anticipated to offer beneficial suppression of T2 and additional inflammatory pathways. The conjectured combined effect in mitigating T2 inflammation may underpin its potential for more effective treatment options for T2 inflammatory diseases.
Dr. Lei Qian, the Clinical Development Vice President at Innovent, remarked, "Over recent years, our comprehension of asthma's pathophysiology, clinical presentation, and treatment avenues has expanded tremendously. We are anticipating the contribution that this novel drug, IBI3002, will make towards asthma and other related inflammatory conditions."
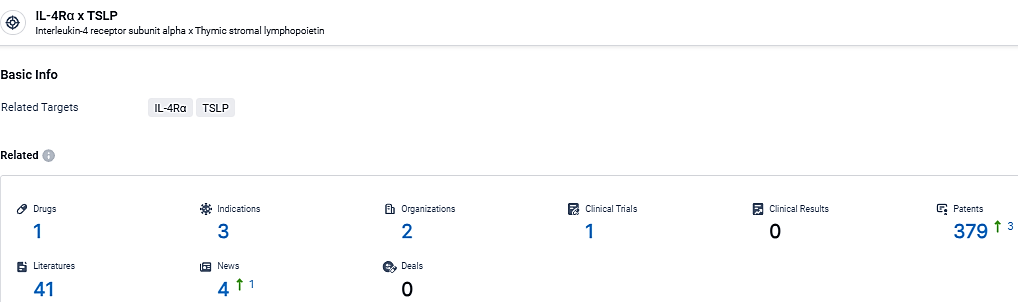
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 4, 2024, there are 1 investigational drugs for the IL-4Rα and TSLP target, including 3 indications, 2 R&D institutions involved, and as many as 379 patents.
IBI-3002 targets the IL-4Rα and TSLP receptors and is currently in Phase 1 of clinical development. As asthma is a prevalent respiratory disease, the development of new therapies like IBI-3002 holds promise for improving patient outcomes and addressing unmet medical needs in this field.