Your Ultimate Guide to Finding Diltiazem on Synapse
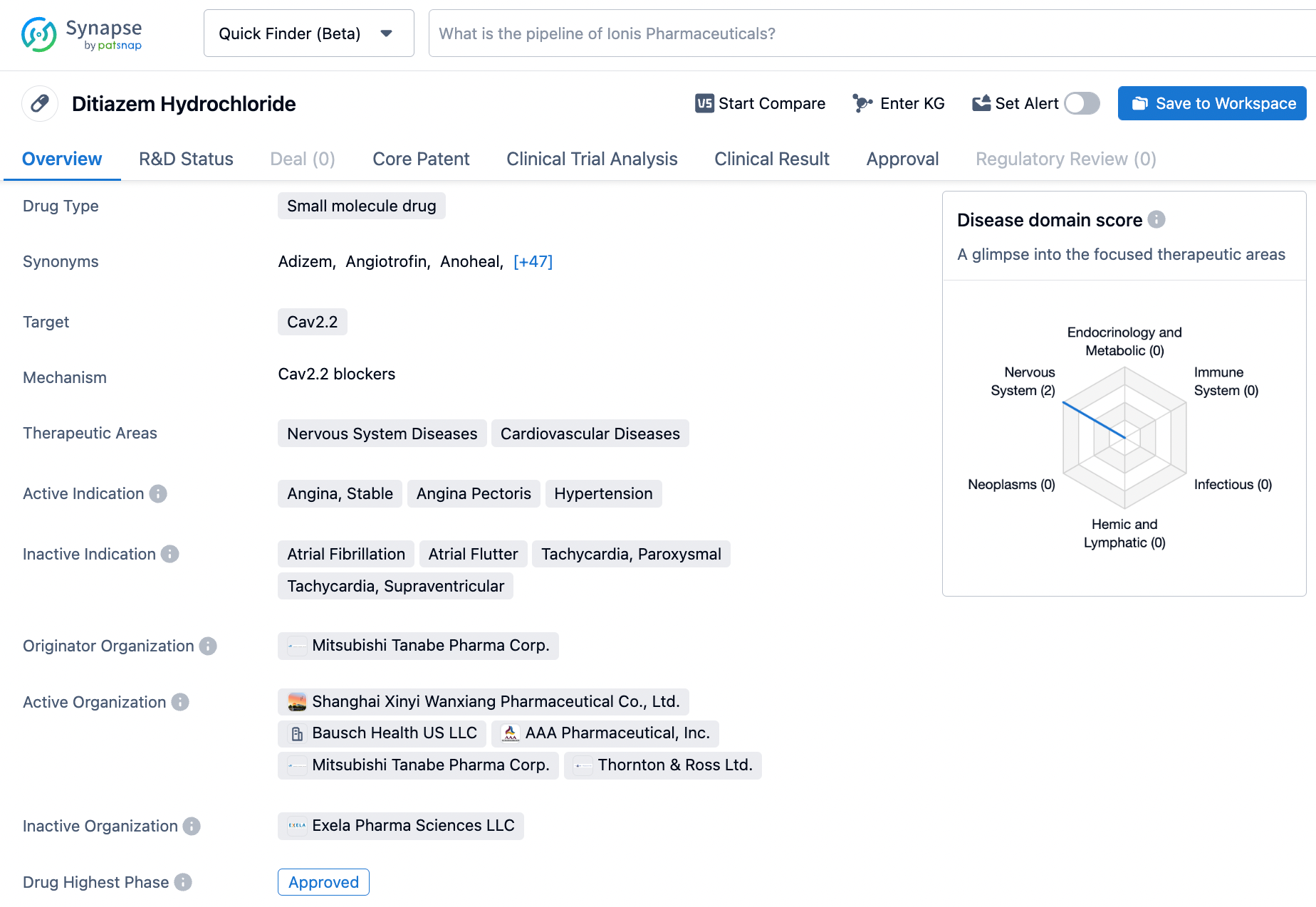
Diltiazem,sold under the brand name Cardizem among others, is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Although precise mechanisms of its antianginal actions are still being delineated, CARDIZEM is believed to act in the following ways:CARDIZEM has been shown to be a potent dilator of coronary arteries both epicardial and subendocardial. Spontaneous and ergonovine-induced coronary artery spasms are inhibited by CARDIZEM. CARDIZEM has been shown to produce increases in exercise tolerance,probably due to its ability to reduce myocardial oxygen demand. This is accomplished via reductions in heart rate and systemic blood pressure at submaximal and maximal exercise workloads. CARDIZEM is indicated for the management of chronic stable angina and angina due to coronary artery spasm. Diltiazem was first launched in Japan by Takeda Mitsubishi in 1973, and later approved by Bausch Health US LLC in the United States in 1982. Click on the image below to begin the exploration journey of Diltiazem through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!