Innovent's SINTBILO® and New Olverembatinib Indication Added to China's National Drug List
Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a leading biopharmaceutical firm specializing in the development, production, and commercialization of high-quality therapies for oncology, cardiovascular, metabolic, autoimmune, ophthalmic, and other significant health conditions, has announced that the revised 2024 National Reimbursement Drug List (NRDL) now features SINTBILO® (tafolecimab injection, an anti-PCSK9 monoclonal antibody) for the first time, along with a new indication for olverembatinib (a BCR-ABL inhibitor). This updated NRDL will officially take effect on January 1, 2025.
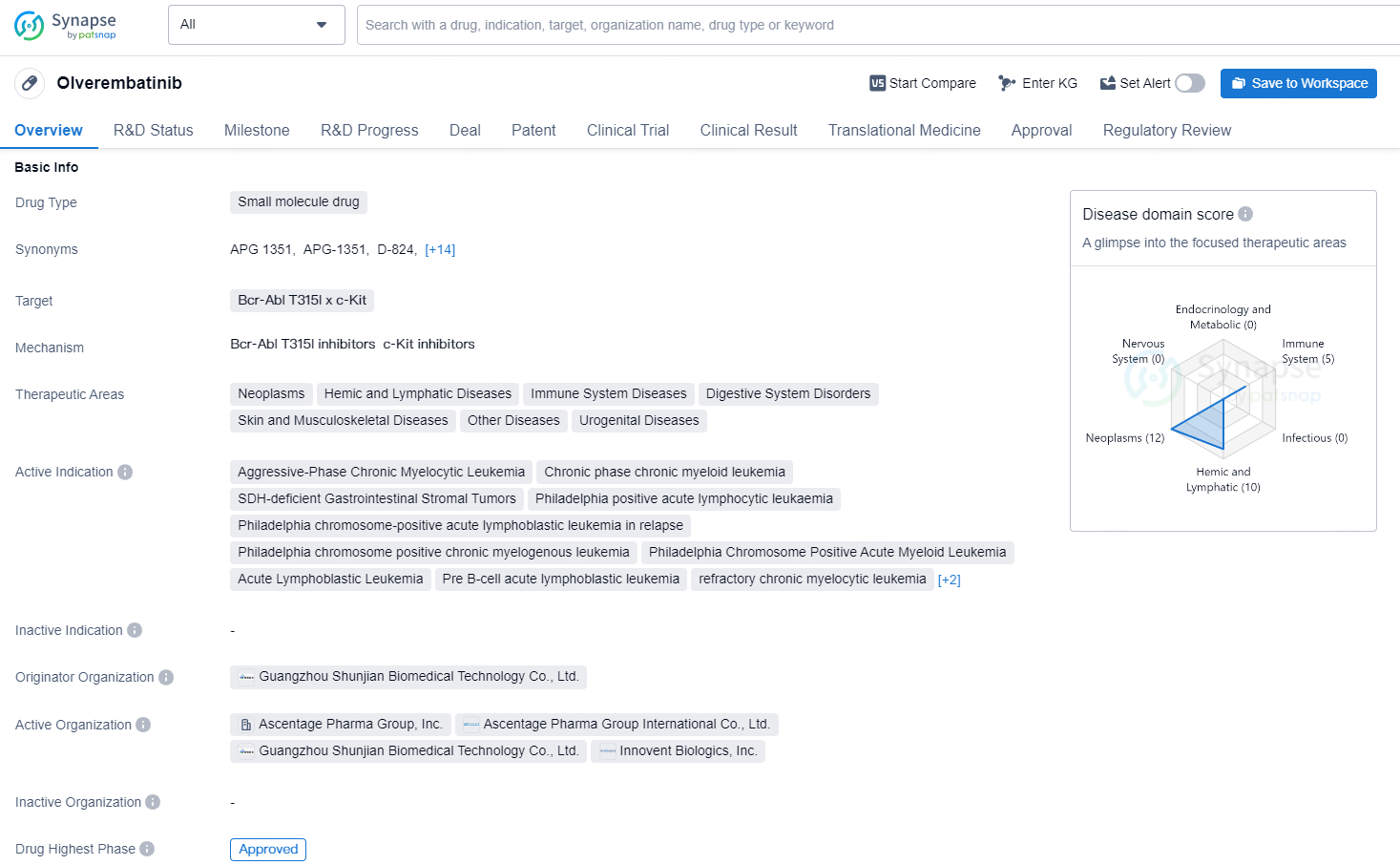
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the revised National Reimbursement Drug List (NRDL), SINTBILO® has been newly added for treating adult patients with primary hypercholesterolemia, which includes both heterozygous familial and non-familial types, as well as mixed dyslipidemia. SINTBILO® (tafolecimab injection) represents Innovent's inaugural venture into cardiovascular medicine, providing various dosing options: 150 mg every two weeks, 450 mg every four weeks, and 600 mg every six weeks. These treatment regimens lead to a substantial decrease in low-density lipoprotein cholesterol (LDL-C) levels by nearly 70% and a reduction in lipoprotein a [Lp(a)] levels by about 50%. Recognized as the first domestic PCSK9 inhibitor included in the NRDL, SINTBILO® presents a crucial new treatment avenue for cholesterol management in China, thereby enhancing the quality of life for many patients suffering from hypercholesterolemia.
Regarding olverembatinib, its original indication was renewed, and an additional new indication was also incorporated into the revised NRDL via a straightforward contract renewal process. The NRDL coverage for olverembatinib encompasses treatment for adult patients with chronic-phase chronic myeloid leukemia (CML-CP) or accelerated-phase CML (CML-AP) with the T315I mutation, as well as for adult patients with CML-CP who are resistant to or intolerant of first- and second-generation tyrosine kinase inhibitors (TKIs). Olverembatinib is recognized as the first third-generation BCR-ABL inhibitor authorized by China's National Medical Products Administration (NMPA). Innovent and Ascentage Pharma are jointly dedicated to bringing olverembatinib to the Chinese market.
Dr. Michael Yu, the Founder, Chairman, and CEO of Innovent, expressed: "We are thrilled about the addition of SINTBILO® to the NRDL, as it significantly enhances access to this innovative therapy, marking a significant milestone for both Innovent and patients dealing with hypercholesterolemia. Additionally, we are heartened by the recognition of olverembatinib's new indication in the NRDL, which will serve a wider array of CML patients. As a company committed to 'empowering patients worldwide with affordable, high-quality biopharmaceuticals', Innovent remains focused on developing cutting-edge treatments across critical fields such as oncology, cardiovascular, and metabolic disorders, autoimmune diseases, and ophthalmology—areas with considerable societal demand. We are eager to continue our patient-focused strategy, capitalizing on our product strengths, and further enhancing drug affordability and accessibility, to ensure that more patients and their families benefit from high-quality medications as swiftly as possible. We take pride in contributing to the improved care of our patients."
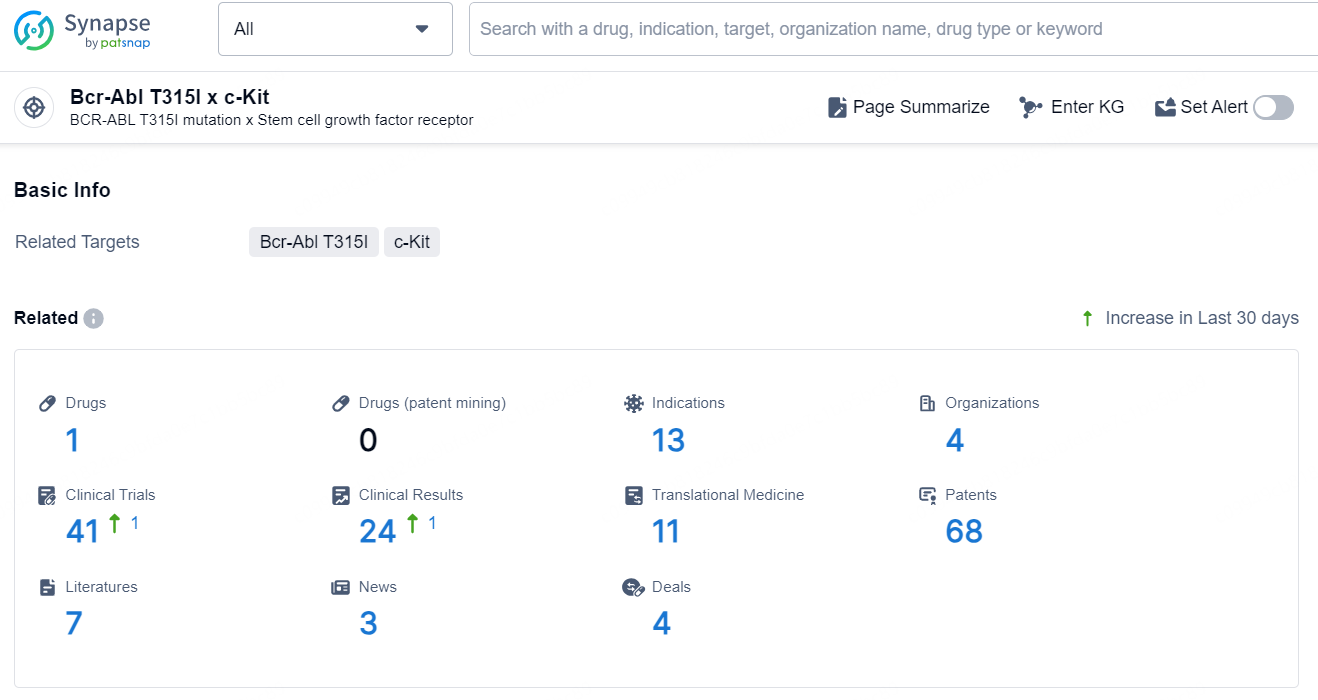
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 28, 2024, there are 1 investigational drug for the Bcr-Abl T315I x c-Kit target, including 13 indications, 4 R&D institutions involved, with related clinical trials reaching 41, and as many as 68 patents.
Olverembatinib is a small molecule drug developed by Guangzhou Shunjian Biomedical Technology Co., Ltd. with approved usage in various therapeutic areas including Neoplasms, Hemic and Lymphatic Diseases, Immune System Diseases, Digestive System Disorders, Skin and Musculoskeletal Diseases, Other Diseases, and Urogenital Diseases. The drug targets Bcr-Abl T315I and c-Kit.