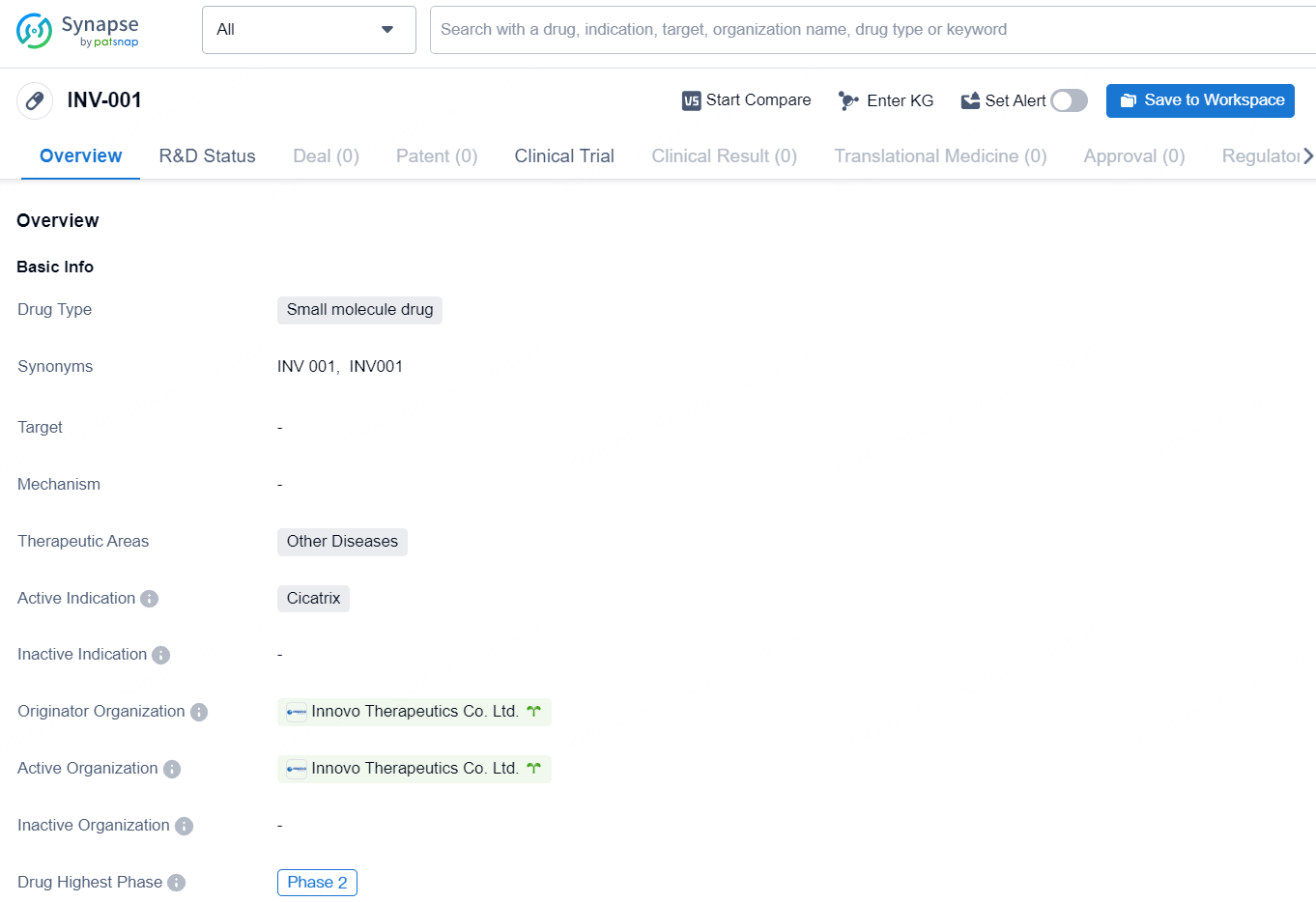
Innovo Therapeutics Unveils Breakthrough Phase 2 Data for Post-Thyroidectomy Scar Treatment INV-001
Innovo Therapeutics Inc. has unveiled the results from its Phase 2 clinical trial, highlighting the novel effectiveness of INV-001 in treating scars. The study included 77 Korean participants with post-thyroidectomy wounds exceeding 3 cm, conducted across four general hospitals, such as Severance Hospital in Seoul. Participants were randomly assigned to start using INV-001 twice a day within 14 days following surgery. The treatment duration was 12 weeks, and its efficacy was evaluated at the 12-week mark using the POSAS (Patient and Observer Scar Assessment Scale).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The trial verified the safety and tolerability of INV-001 at both 0.2% and 2% doses, with no serious adverse events (SAEs) reported. A statistically significant difference (p<0.05, Analysis of Covariance - ANCOVA) was noted in the group that received the clinical drug in a 2% dose for 12 weeks, showing a 24.5% reduction in scars compared to the placebo, as per the per-protocol set.
Key Findings:
Primary Endpoint: The primary efficacy endpoint was the Overall Opinion score on the POSAS: Observer scale at 12 weeks. Lower scores were observed in all treatment groups compared to the placebo. The high-dose (2%) treatment group also exhibited a statistically significant reduction in score from baseline to 12 weeks.
Secondary Endpoints: In the secondary efficacy analysis, there was a trend toward scar improvement at 3 and 6 weeks, but no statistically significant differences were observed. The high-dose (2%) treatment group showed gradual improvement over time, with differences in scar improvement compared to the placebo becoming more pronounced. POSAS Patient scale scores showed improvement trends at 3, 6, and 12 weeks, with differences between the placebo and both the low-dose (0.2%) and high-dose (2%) treatment groups exceeding the Minimal Clinically Important Difference (MCID) of 0.39, indicating clinically meaningful differences.
Pharmacokinetic Analysis: The average blood concentration of INV-001 in the high-dose (2%) treatment group remained below 3.6 ng/mL at 3 weeks, and this concentration stayed consistent at 6 and 12 weeks.
Safety Results: Safety evaluations revealed that all reported adverse drug reactions (ADRs) were predictable and occurred at the application site of the clinical trial drug. There were no serious adverse events (SAEs) nor serious ADRs (SADRs).
Professor Won-Jae Lee, the Coordinating Investigator and a plastic surgeon at Yonsei University Severance Hospital, mentioned that this clinical trial is the first in the world to confirm the scar suppression effects of HSP47 inhibition. He highlighted the promising scar reduction effects of INV-001. Additionally, he pointed out that due to the current lack of specialized topical medications for scar suppression, the development of a specialized ointment from this clinical trial would be greatly beneficial for both clinicians and patients.
Dr. Hee Dong Park, a founder and CEO of Innovo Therapeutics, stated that this clinical trial has demonstrated the safety and efficacy of INV-001, underlining its potential as a scar treatment. He emphasized that its innovative mechanism has the potential to provide significant value to patients in the scar treatment market.
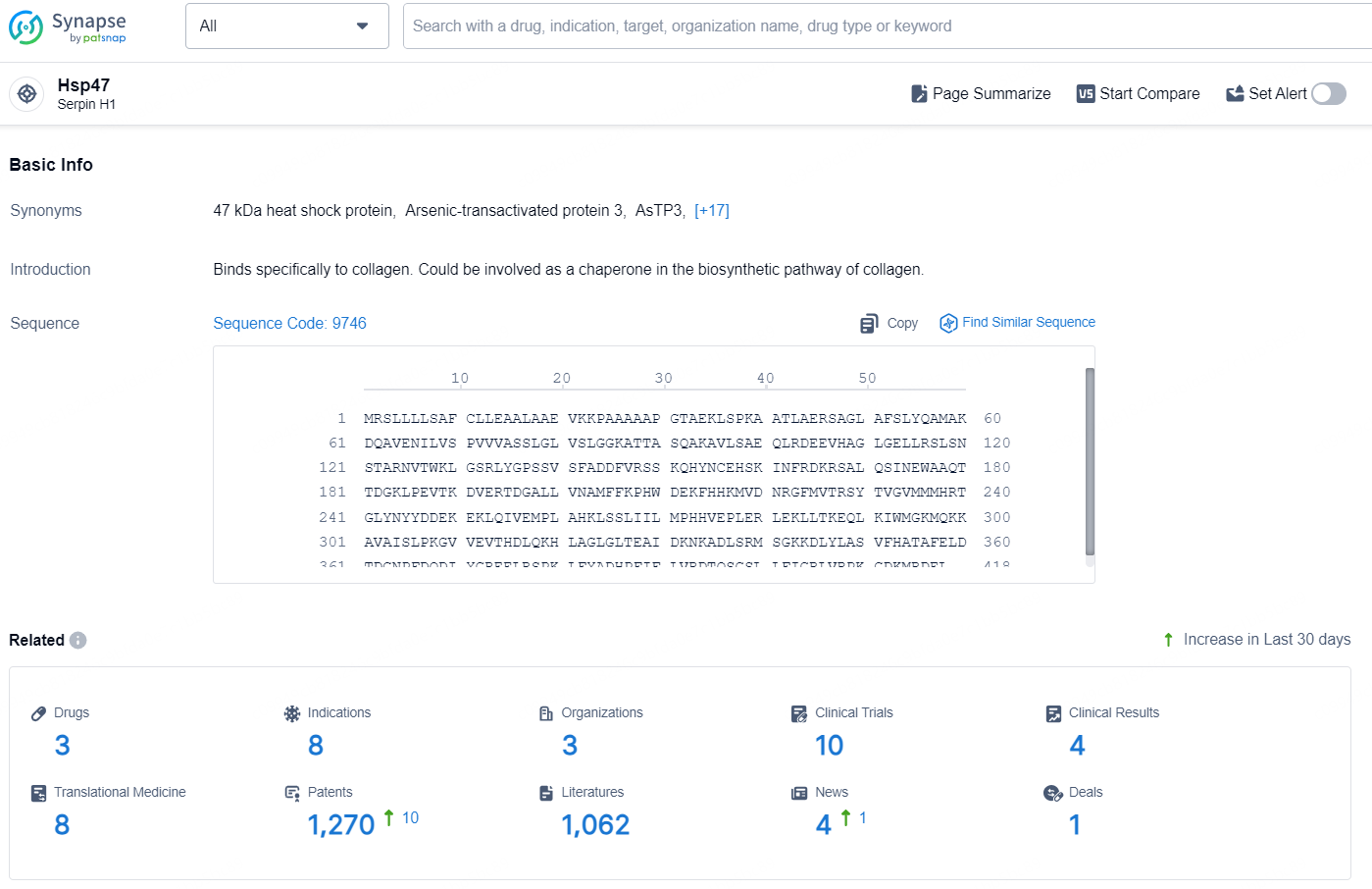
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 23, 2024, there are 3 investigational drugs for the HSP47 target, including 8 indications, 3 R&D institutions involved, with related clinical trials reaching 10, and as many as 1270 patents.
INV-001 inhibits HSP47, a key protein in collagen formation, transport, and extracellular secretion, thereby preventing and treating post-surgical and trauma-induced scars. Results from disease animal models have confirmed that INV-001 effectively inhibits trauma-induced scars without affecting wound healing.