U.S. Approves RYBREVANT® and LAZCLUZE™ for Initial, Chemotherapy-Free Treatment of Advanced Lung Cancer with EGFR Mutations
Johnson & Johnson (NYSE: JNJ) revealed today that the U.S. Food and Drug Administration (FDA) has given the green light to RYBREVANT® (amivantamab-vmjw) in combination with LAZCLUZE™ (lazertinib) for the initial treatment of adult individuals with locally advanced or metastatic non-small cell lung cancer (NSCLC) featuring epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations, identifiable via an FDA-approved test.
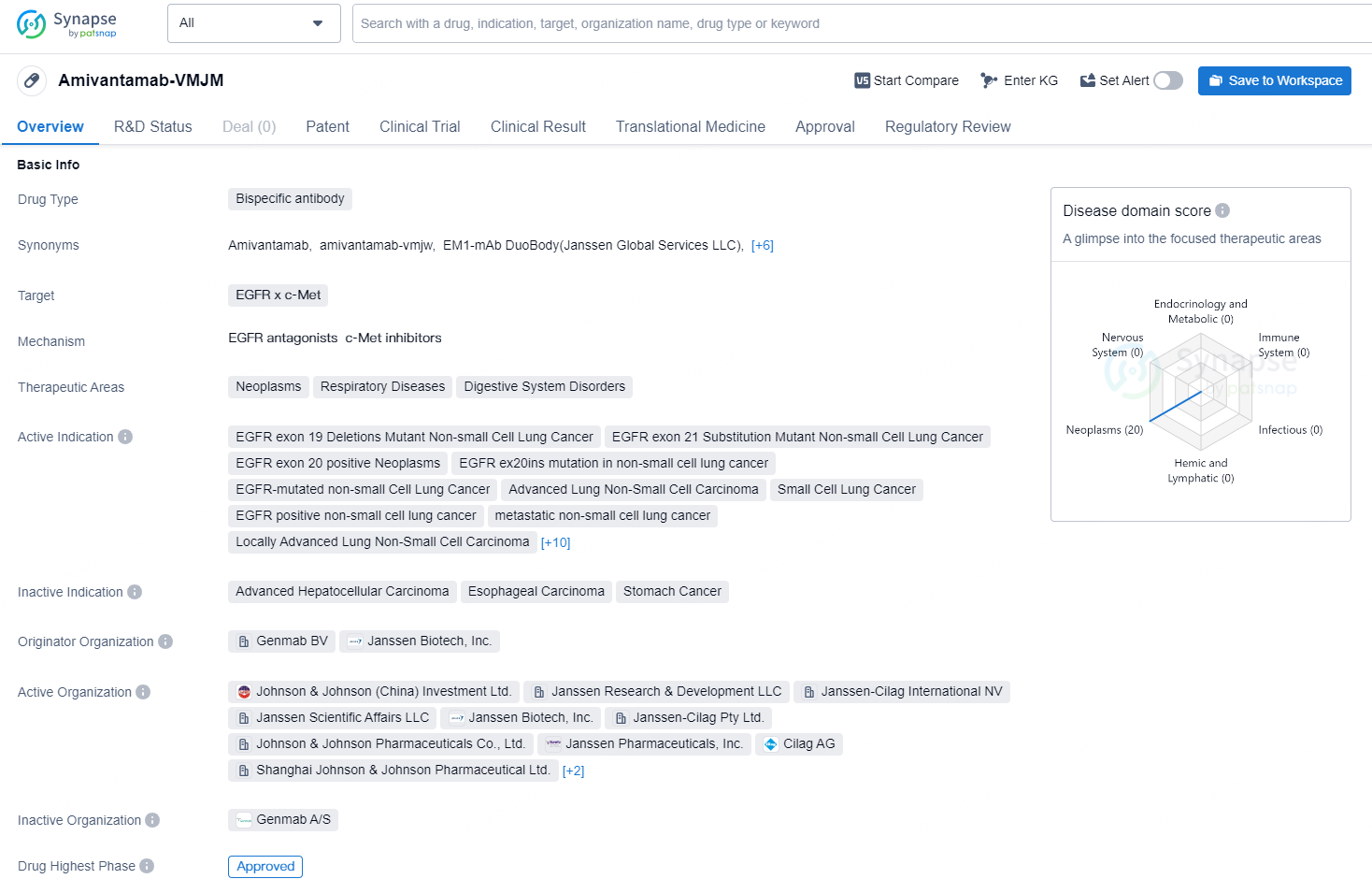
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
With this achievement, RYBREVANT® combined with LAZCLUZE™ has become the first and only multi-targeted, chemotherapy-free combination therapy to show superiority over osimertinib for the initial treatment of patients with EGFR-mutated NSCLC. RYBREVANT® is a bispecific antibody that targets EGFR- and MET* and activates the immune system, whereas LAZCLUZE™ is a highly selective, brain-penetrating, third-generation oral EGFR TKI**. This combination, RYBREVANT® plus LAZCLUZE™, uniquely targets both common EGFR mutations directly.
“This approval marks a significant advancement for patients with EGFR-mutated NSCLC, addressing long-standing unmet needs,” said Jill Feldman†, a lung cancer survivor and co-founder of the EGFR Resisters, a patient advocacy organization. “Having observed the significant progress in lung cancer treatments firsthand, this milestone introduces a new therapeutic strategy for patients and their families. I’m excited that more patients can now benefit from the progression-free survival improvements demonstrated in the MARIPOSA study.”
Lung cancer remains the leading cause of cancer death globally, accounting for 1.8 million deaths annually, with NSCLC representing 80 to 85 percent of all lung cancer cases. Among those with EGFR-mutated NSCLC, 25 to 39 percent do not receive second-line therapy due to disease progression and limited treatment options. The five-year survival rate is below 20 percent for individuals with advanced EGFR-mutated NSCLC treated with the current standard TKI monotherapy, and acquired resistance mechanisms following TKI monotherapy complicate subsequent treatments.
“The distinct combination of RYBREVANT and LAZCLUZE has shown superior efficacy in first-line treatment of specific patients with advanced EGFR-mutated NSCLC, as evidenced by the MARIPOSA study,” said Alexander Spira‡, M.D., Ph.D., FACP, Director of the Virginia Cancer Specialists Research Institute, and a study investigator. “Patients now have a potential new first-line standard of care that offers significant clinical benefits over osimertinib. This targeted first-line therapy aims to achieve the best possible outcomes for patients while reserving chemotherapy for later stages of treatment when resistance becomes more challenging.”
The FDA's approval is based on positive outcomes from the Phase 3 MARIPOSA study, which demonstrated that RYBREVANT® plus LAZCLUZE™ reduced the risk of disease progression or death by 30 percent compared to osimertinib (median progression-free survival [PFS]: 23.7 months versus 16.6 months) in the first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations. Additionally, the median duration of response (DOR) was nine months longer with RYBREVANT® plus LAZCLUZE™ compared to osimertinib (25.8 months versus 16.7 months), a secondary endpoint of the study.
“Leveraging over three decades of oncology innovation, we are uniquely poised to develop best-in-class treatments where survival rates have been stagnant for years,” said Jennifer Taubert, Executive Vice President and Worldwide Chairman of Innovative Medicine at Johnson & Johnson. “RYBREVANT plus LAZCLUZE sets a new standard in advanced first-line therapy, and we are eager to bring this new chemotherapy-free regimen to patients.”
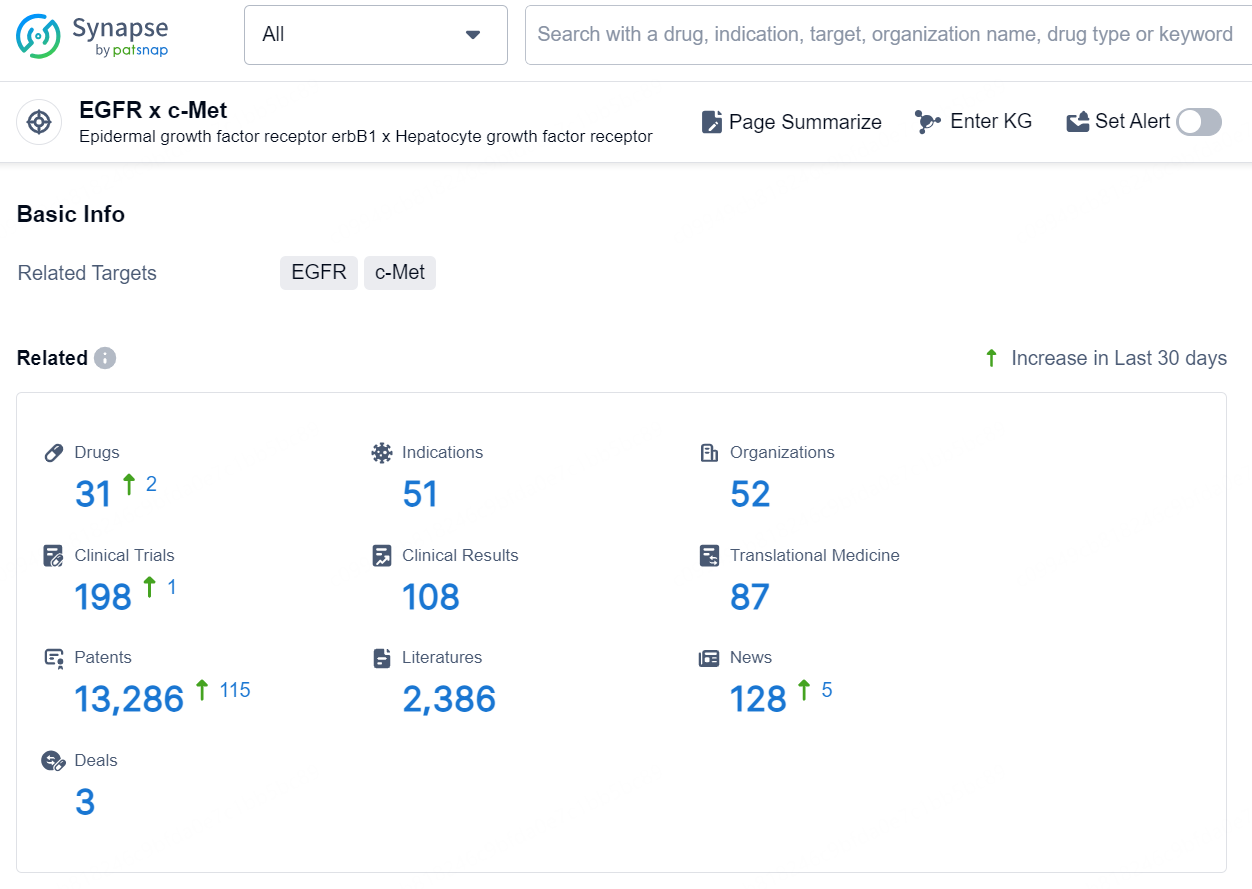
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 23, 2024, there are 31 investigational drugs for the EGFR x c-Met target, including 51 indications, 52 R&D institutions involved, with related clinical trials reaching 198, and as many as 13286 patents.
Amivantamab-VMJM is a bispecific antibody drug developed by Genmab BV and Janssen Biotech, Inc. It targets both EGFR and c-Met and is indicated for the treatment of various neoplasms, respiratory diseases, and digestive system disorders. The drug is specifically approved for the treatment of several types of non-small cell lung cancer (NSCLC), including EGFR exon 19 deletions mutant NSCLC, EGFR exon 21 substitution mutant NSCLC, and EGFR exon 20 positive neoplasms. It is also indicated for the treatment of advanced lung NSCLC, small cell lung cancer, metastatic solid tumor, gastrointestinal neoplasms, colorectal cancer, and squamous cell carcinoma of the head and neck, among others.