Instil Bio and ImmuneOnco Partner for IMM2510 and IMM27M Development
Instil Bio, Inc. and ImmuneOnco Biopharmaceuticals Inc. have disclosed a binding agreement where Instil will acquire the rights to develop and commercialize ImmuneOnco’s proprietary PD-L1xVEGF bispecific antibody, IMM2510, and its next-generation anti-CTLA-4 antibody, IMM27M, outside of China.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
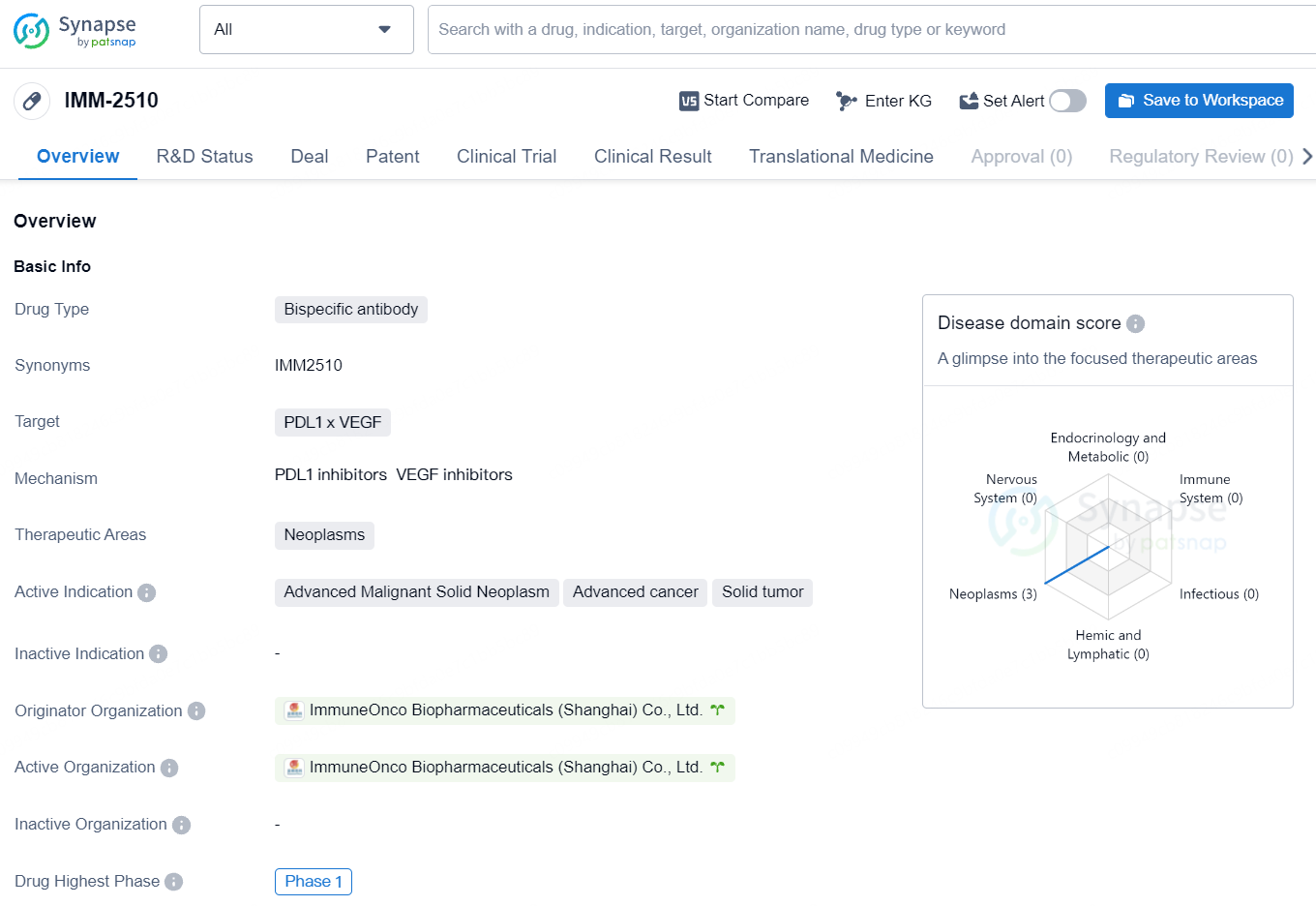
IMM2510 is a groundbreaking bispecific antibody, combining an anti-PD-L1 antibody with a vascular endothelial growth factor receptor (VEGFR) “trap” that captures VEGF. This antibody stands out from other PD(L)1xVEGF antibodies through its ability to bind a range of VEGF receptor ligands beyond VEGF-A, its smaller size which may improve tumor infiltration, and its enhanced antibody-dependent cellular cytotoxicity (ADCC) aimed at increasing tumor destruction.
IMM2510 has finished a dose-escalation clinical trial for advanced solid tumors, showing several responses, including in patients with squamous non-small cell lung cancer who were previously unresponsive to PD-1 inhibitors.
IMM27M is a next-generation anti-CTLA-4 antibody, featuring increased ADCC activity, and engineered to deplete intratumoral regulatory T cells. This is intended to enhance treatment efficacy and lower the toxicity linked with first-generation anti-CTLA-4 antibodies. IMM27M has concluded a dose-escalation clinical study that demonstrated anti-tumor activity in patients with advanced solid tumors. It commenced combination studies with IMM2510 in China in July 2024.
Per the agreement terms, Instil’s wholly owned subsidiary will obtain global development and commercialization rights for IMM2510 and IMM27M outside of Greater China, while ImmuneOnco will retain these rights within Greater China, encompassing Taiwan, Macau, and Hong Kong. ImmuneOnco will receive an upfront payment and potential near-term payments up to $50 million, along with potential additional development, regulatory, and commercial milestone payments exceeding $2 billion, plus single-digit to low double-digit percentage royalties on ex-China global sales.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
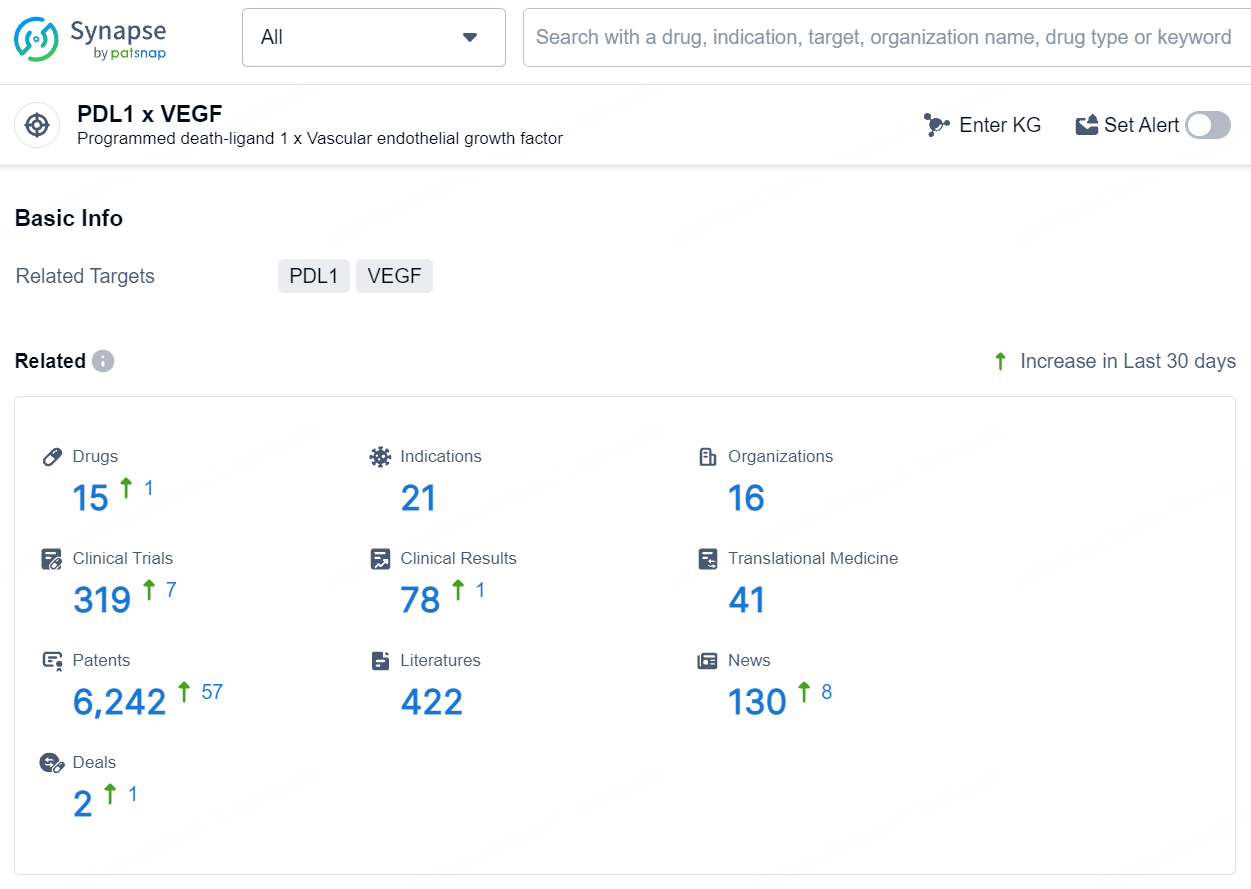
According to the data provided by the Synapse Database, As of August 7, 2024, there are 15 investigational drugs for the PD-L1 and VEGF target, including 21 indications, 16 R&D institutions involved, with related clinical trials reaching 319, and as many as 6242 patents.
IMM-2510 is a bispecific antibody that targets PDL1 and VEGF and is being developed for the treatment of advanced malignant solid neoplasms, advanced cancer, and solid tumors. The drug has reached Phase 1 of clinical development, both globally and in China, indicating its potential as a novel therapy for these conditions.