RegenxBio reveals promising new results from RGX-202 AFFINITY DUCHENNE® study
REGENXBIO Inc. announced fresh, encouraging interim results on safety and efficacy from the Phase I/II AFFINITY DUCHENNE trial of RGX-202 in Duchenne muscular dystrophy patients aged 1 to 11 years.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
RGX-202 is an experimental AAV Therapy created to deliver a novel microdystrophin gene that comprises essential regions of the natural dystrophin gene. It is the sole gene therapy approved or currently under development for Duchenne that includes the C-Terminal domain, aiming to produce a microdystrophin more similar to the naturally occurring form. In preclinical trials, the CT domain has demonstrated its ability to shield muscle from stress induced by contraction and enhance its self-repair capabilities.
"We are observing a notable dose response and uniform, impressive levels of microdystrophin expression among all treated patients in the AFFINITY DUCHENNE trial, with particularly high expression reported in older ambulatory subjects," stated Curran M. Simpson, President and CEO of REGENXBIO.
"This data continues to augment the growing body of evidence supporting RGX-202's potential as a standout, leading-edge treatment for Duchenne. RGX-202 is well-positioned to become the next approved gene therapy for Duchenne, and with our commercial-ready, suspension-based manufacturing process, we believe we can meet the demand of the entire market," added Curran M. Simpson.
"I'm encouraged by the biomarker results from the AFFINITY DUCHENNE study of RGX-202 and am looking forward to the initial functional data from this program," commented Aravindhan Veerapandiyan, M.D., from Arkansas Children's Hospital. "This update is also promising for the Duchenne community, which is investigating various treatments that might affect disease progression."
RGX-202 is a next-generation experimental gene therapy aimed at enhancing function and outcomes in Duchenne. It is the only gene therapy approved or in late-stage development for Duchenne, boasting a unique microdystrophin construct that includes significant regions of natural dystrophin, such as the C-Terminal domain. In preclinical tests, the CT domain has proven to protect muscles from contraction-induced stress and aid in self-repair.
Additional design elements, like codon optimization and reduced CpG content, potentially enhance gene expression, increase protein translation efficiency, and lower immunogenicity. RGX-202 is crafted to promote the delivery and targeted expression of genes throughout skeletal and cardiac muscle, utilizing the NAV AAV8 vector and a well-established muscle-specific promoter.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
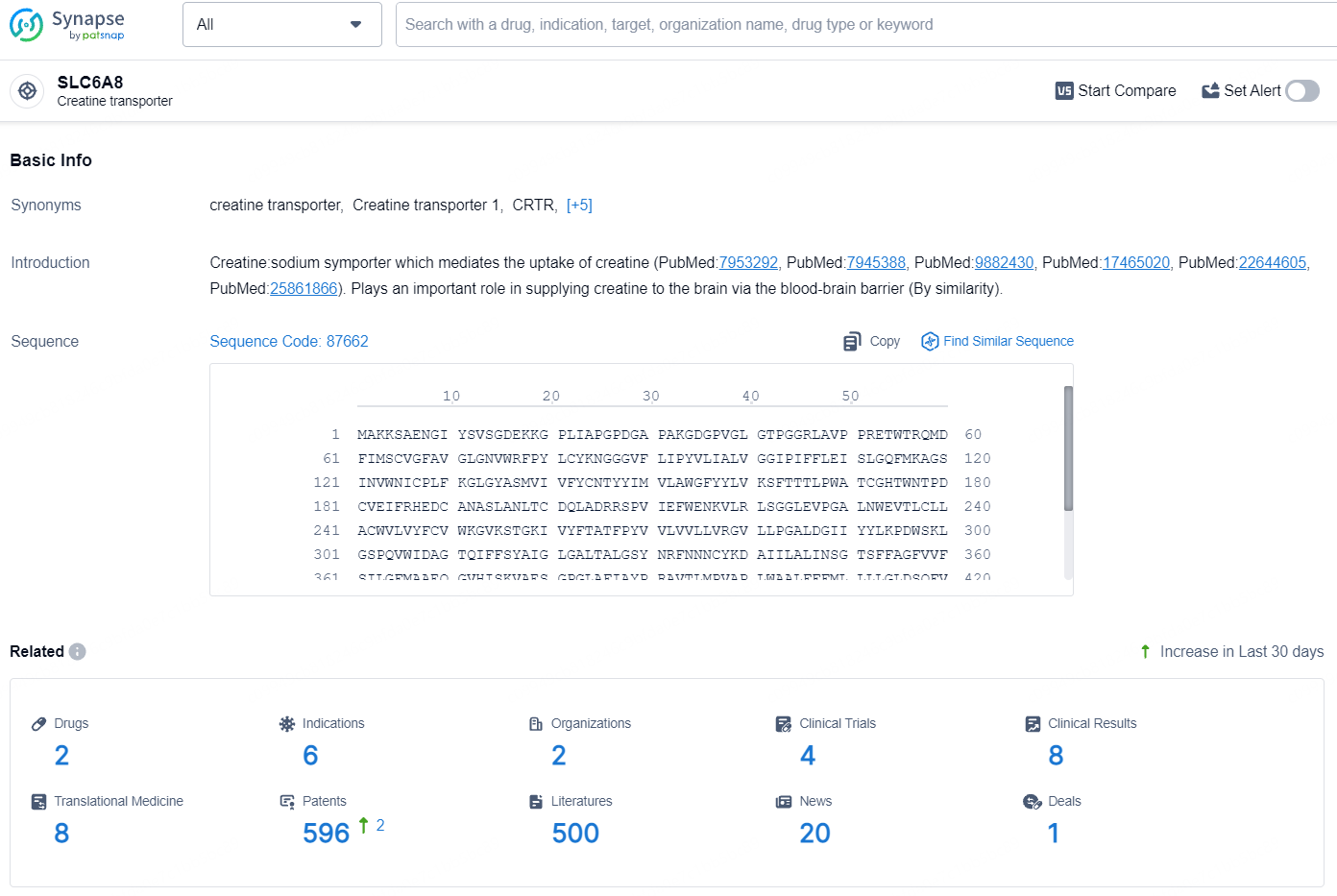
According to the data provided by the Synapse Database, As of August 6, 2024, there are 2 investigational drugs for the SLC6A8 target, including 6 indications, 2 R&D institutions involved, with related clinical trials reaching 4, and as many as 596 patents.
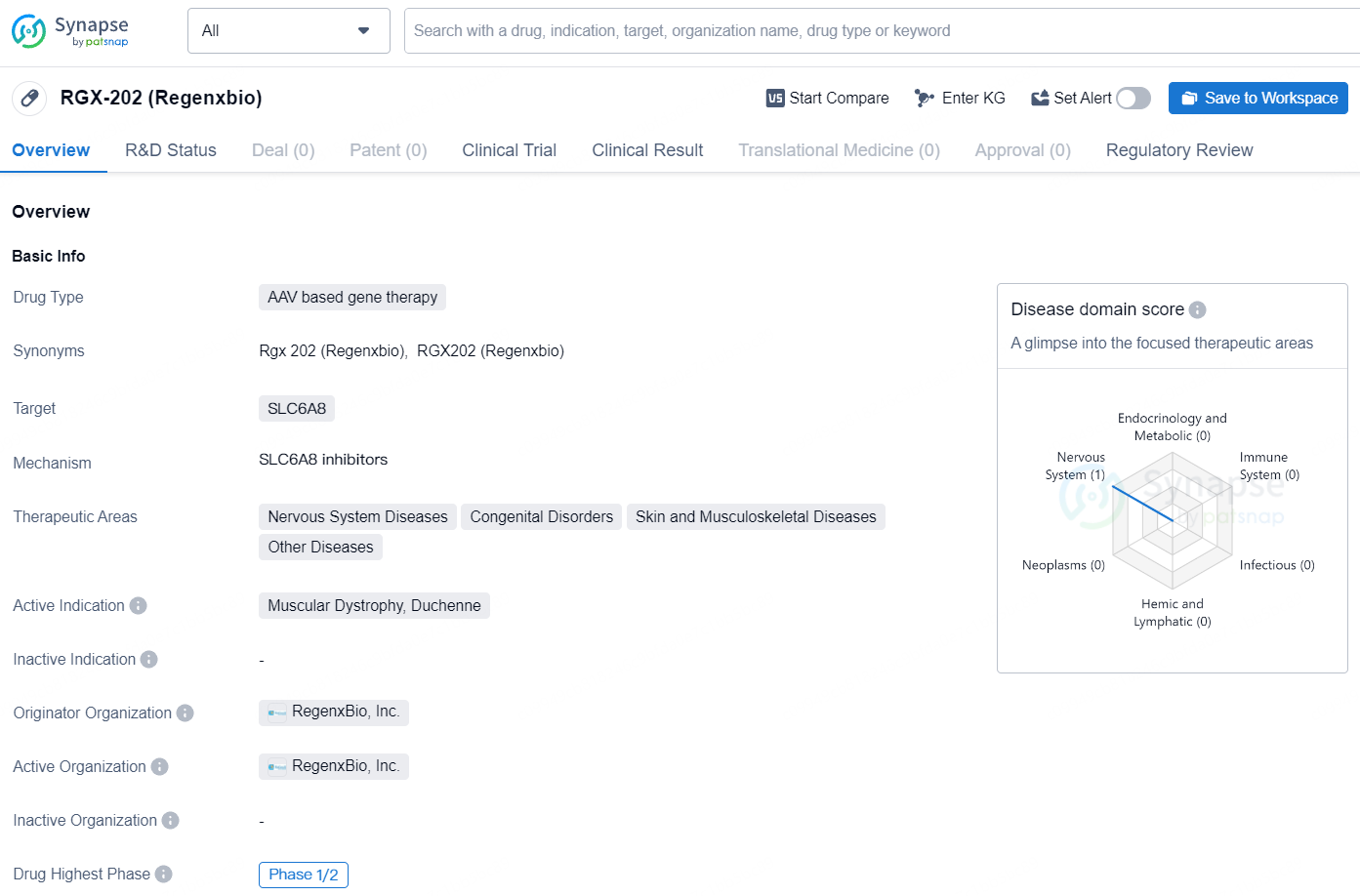
RGX-202 by Regenxbio is a gene therapy drug with a focus on treating muscular dystrophy, particularly Duchenne muscular dystrophy. Its AAV based gene therapy targets SLC6A8 and has the potential to address a range of therapeutic areas including nervous system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug has been given special regulatory designations including Rare Pediatric Disease, Fast Track, and Orphan Drug status, highlighting its potential to provide benefits to patients with rare or serious medical conditions. With its current highest phase of Phase 1/2, RGX-202 represents a promising development in the field of biomedicine and gene therapy.