Is Motixafortide approved by the FDA?
Motixafortide, marketed under the brand name Aphexda, was approved by the FDA on September 8, 2023. Motixafortide is a hematopoietic stem cell mobilizer used in combination with another medicine to increase the number of stem cells for collection before a stem cell transplant in adults with multiple myeloma.
Uses
Motixafortide is primarily used to treat adults with multiple myeloma by aiding in the mobilization of hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation.
Administration
Motixafortide is administered via a subcutaneous injection by a healthcare professional. The dosing is based on the patient's weight, with the usual dose being 1.25 mg/kg given as a slow injection approximately 10 to 14 hours prior to the initiation of the first apheresis. Premedication is recommended to reduce the risk of hypersensitivity and injection site reactions.
Side Effects
Common side effects of motixafortide may include:
- Pain, bruising, swelling, redness, itching, or irritation at the injection site
- Flushing
- Back pain
Serious side effects can occur and may include:
- Chills
- Vomiting, nausea
- Itching, rash
- Flushing (sudden warmth, redness, or tingly feeling)
Patients should get emergency medical help if they experience signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Warnings and Precautions
- Motixafortide should not be used in people with leukemia.
- It may harm an unborn baby, so effective birth control is necessary during treatment and for at least 8 days after the last dose.
- Breastfeeding should be avoided while using motixafortide and for at least 8 days after the last dose.
Interactions
Patients should inform their doctor about all other medications they are using, including prescription, over-the-counter medicines, vitamins, and herbal products, as they may affect motixafortide.
Conclusion
Motixafortide (Aphexda) is FDA-approved and plays a significant role in the treatment regimen for multiple myeloma by mobilizing stem cells for collection and transplantation. The approval date is September 8, 2023, and it is crucial for patients to follow all medical guidelines and report any adverse effects to their healthcare provider.
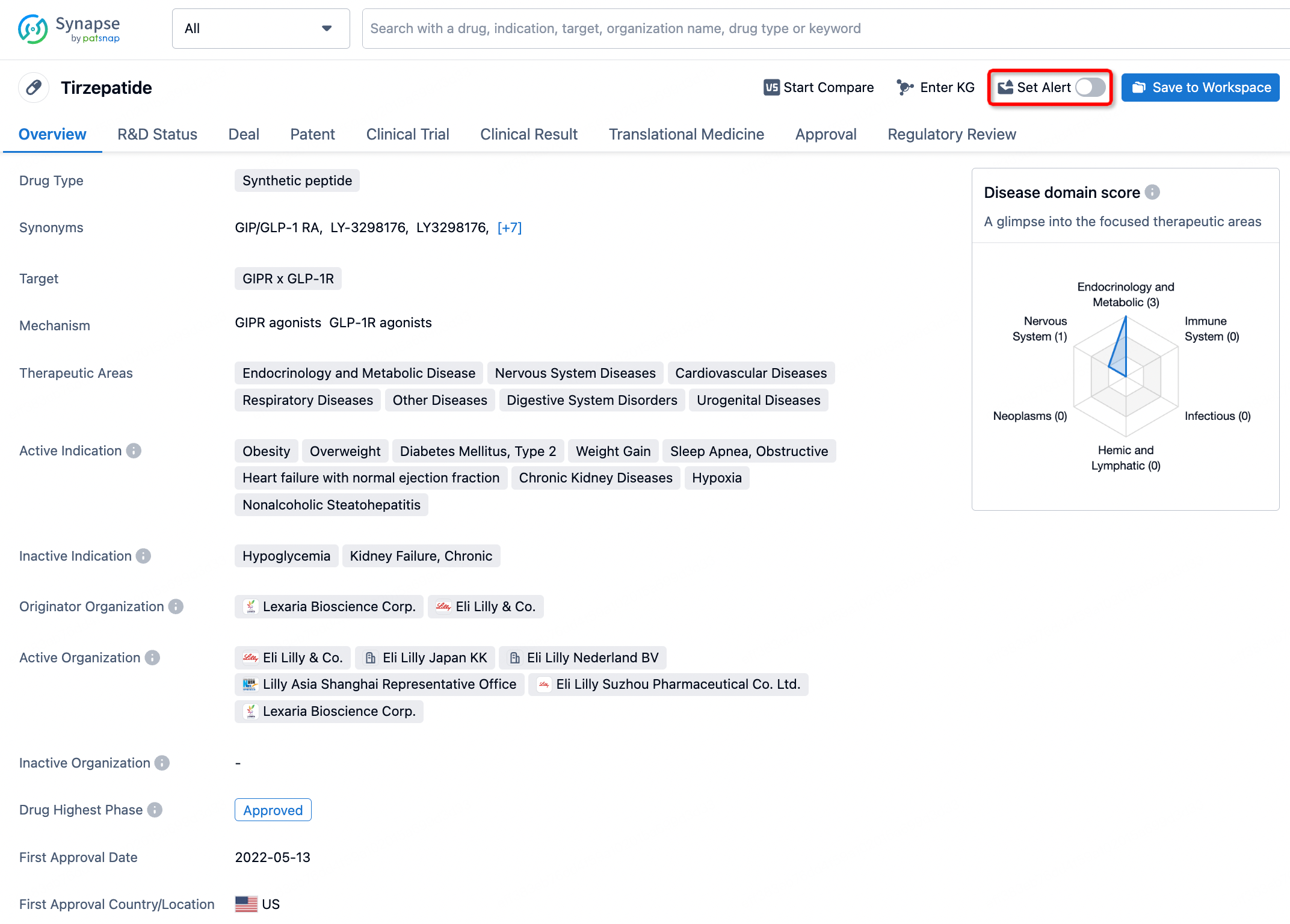
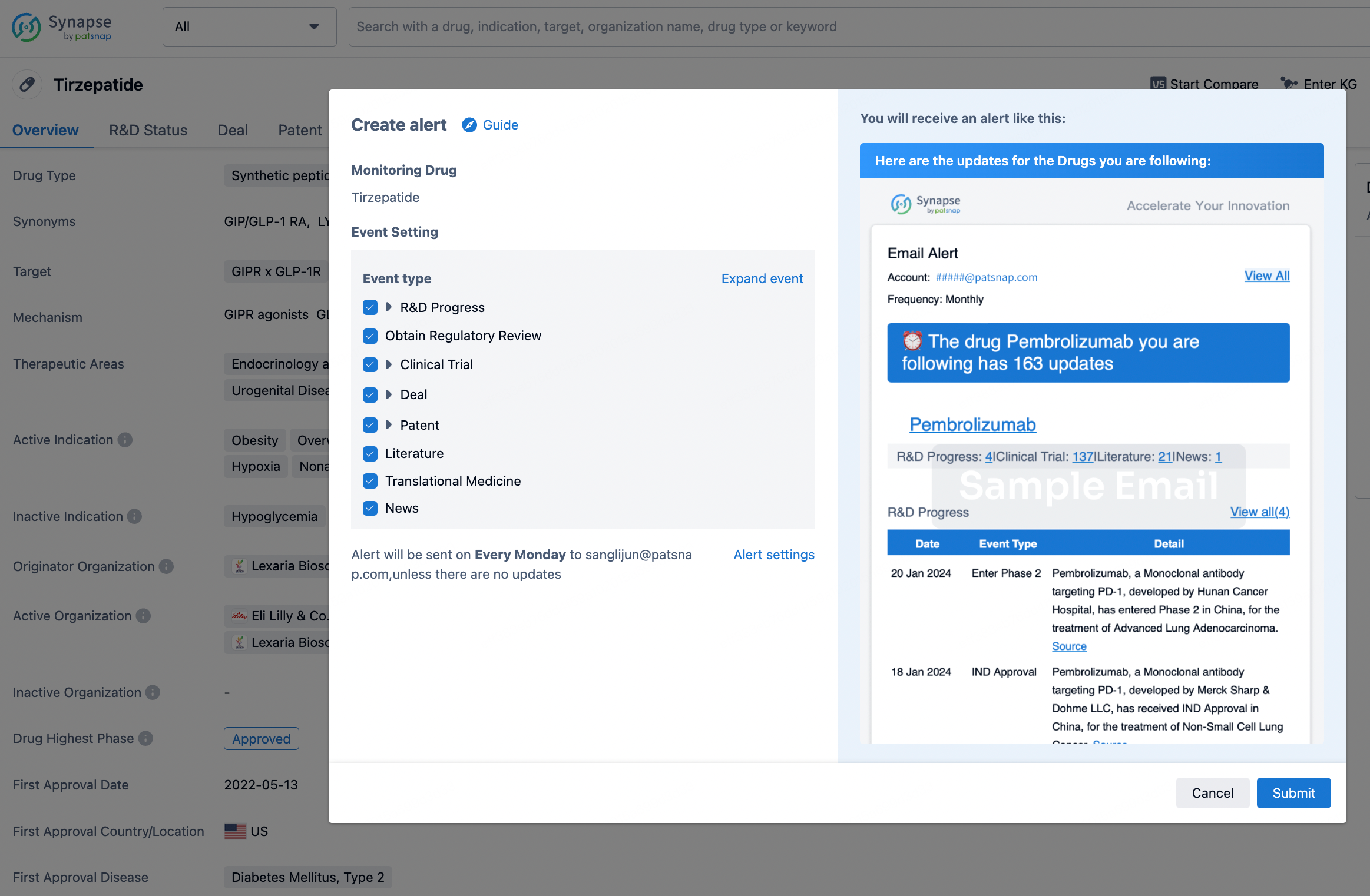
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!