iOmx Therapeutics Commences Phase Ib Trial Featuring OMX-0407
iOmx Therapeutics AG (iOmx), a biopharmaceutical company at the clinical stage, focused on translating under-explored immune evasion biology into an expanding portfolio of biomarker-enabled drug programs, has announced the successful completion of the dose escalation portion of the Phase Ia/Ib clinical trial (NCT05826600). This trial is investigating OMX-0407, a first-in-class, spectrum-selective SIK (salt-inducible kinase) inhibitor, in patients with advanced solid tumors. The expansion phase of the study is now open for kidney cancer and angiosarcoma.
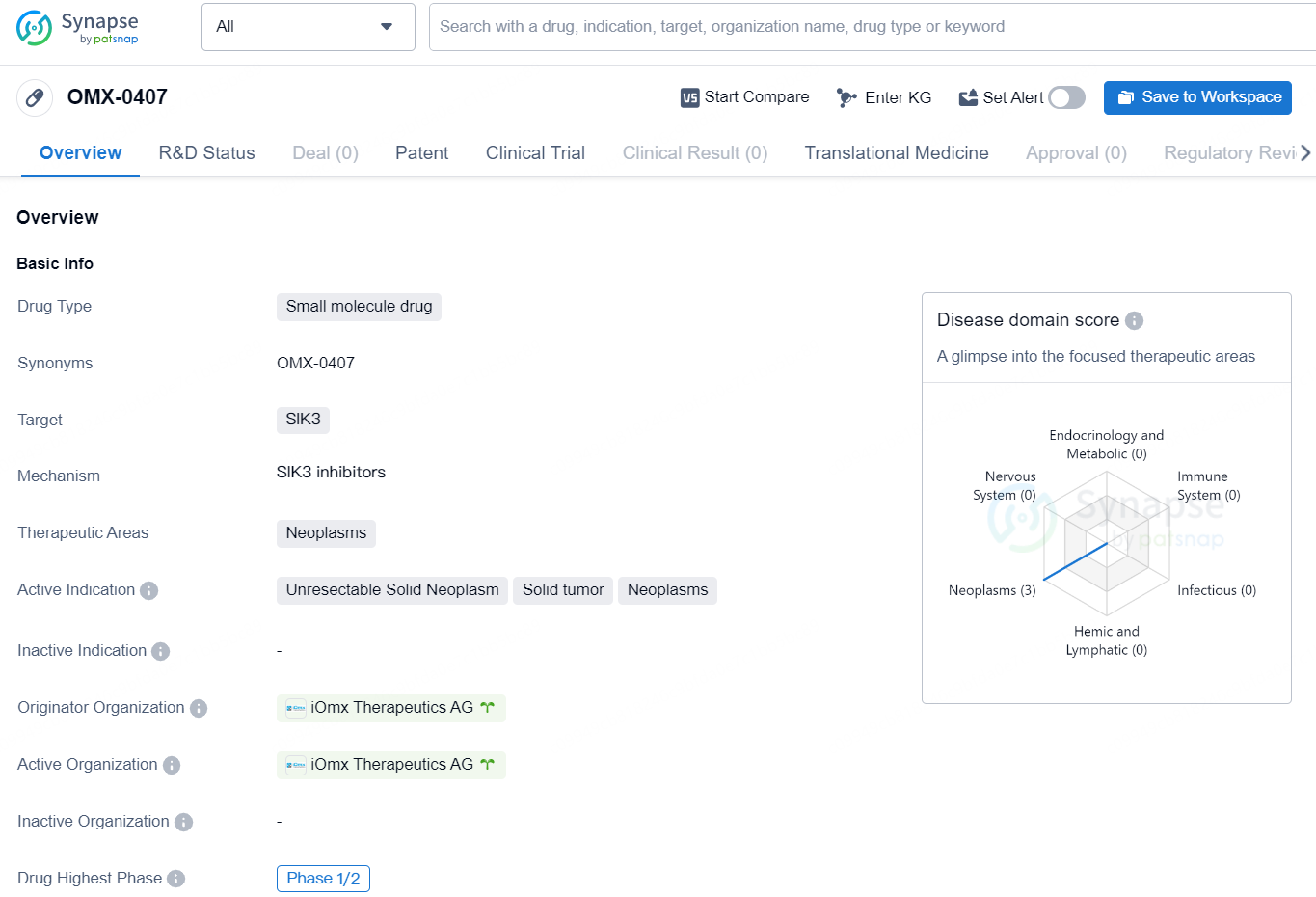
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Murray Yule, M.D., Chief Medical Officer at iOmx, commented, “Concluding the dose escalation phase of our Phase Ia/Ib clinical trial represents a crucial advancement in the development of our primary candidate, OMX-0407. I am eager to see the beneficial impact OMX-0407 will have on broader patient groups, particularly those with kidney cancer and angiosarcoma.”
Initial data from the 20-patient dose escalation phase demonstrated that OMX-0407 monotherapy is both safe and well-tolerated. Anti-cancer activity was evident among this heavily pretreated group of patients with various solid tumors. Remarkably, one patient resistant to multiple prior chemotherapies achieved a durable complete response (CR), with all tumor lesions disappearing. Moreover, biomarker analyses revealed significant pharmacological activity in circulating blood cells.
Hannes Loferer, Ph.D., Chief Operations Officer at iOmx, stated, “Our current focus is on generating efficacy data in the dose expansion segment of the trial. Given the results from the dose escalation and preclinical data, kidney cancer and angiosarcoma, among other prioritized key solid tumor indications, show high potential for an objective response to OMX-0407.”
The expansion cohorts will be conducted at leading oncology centers specializing in kidney cancer and angiosarcoma. This phase aims to further assess the monotherapy activity and the safety profile of OMX-0407 at the recommended dose established in the completed dose escalation phase. Supported by a strong translational data package, the study will enroll patients with previously treated renal cell carcinoma and angiosarcoma. The primary endpoint is the objective response rate, with secondary objectives including the duration of response and patient survival.
Apollon Papadimitriou, Ph.D., Chief Executive Officer at iOmx, concluded, “The initiation of the Phase Ib segment of the OMX-0407 trial marks a major milestone for iOmx. The dose escalation data, which includes a long-lasting complete response in an area of high medical need and additional signs of anti-tumor activity in other patients, is very promising. We believe we are on the right path towards delivering a clinically meaningful, distinct therapy for patients not responding to current cancer treatments and expect to release topline clinical proof-of-concept data by early 2026.”
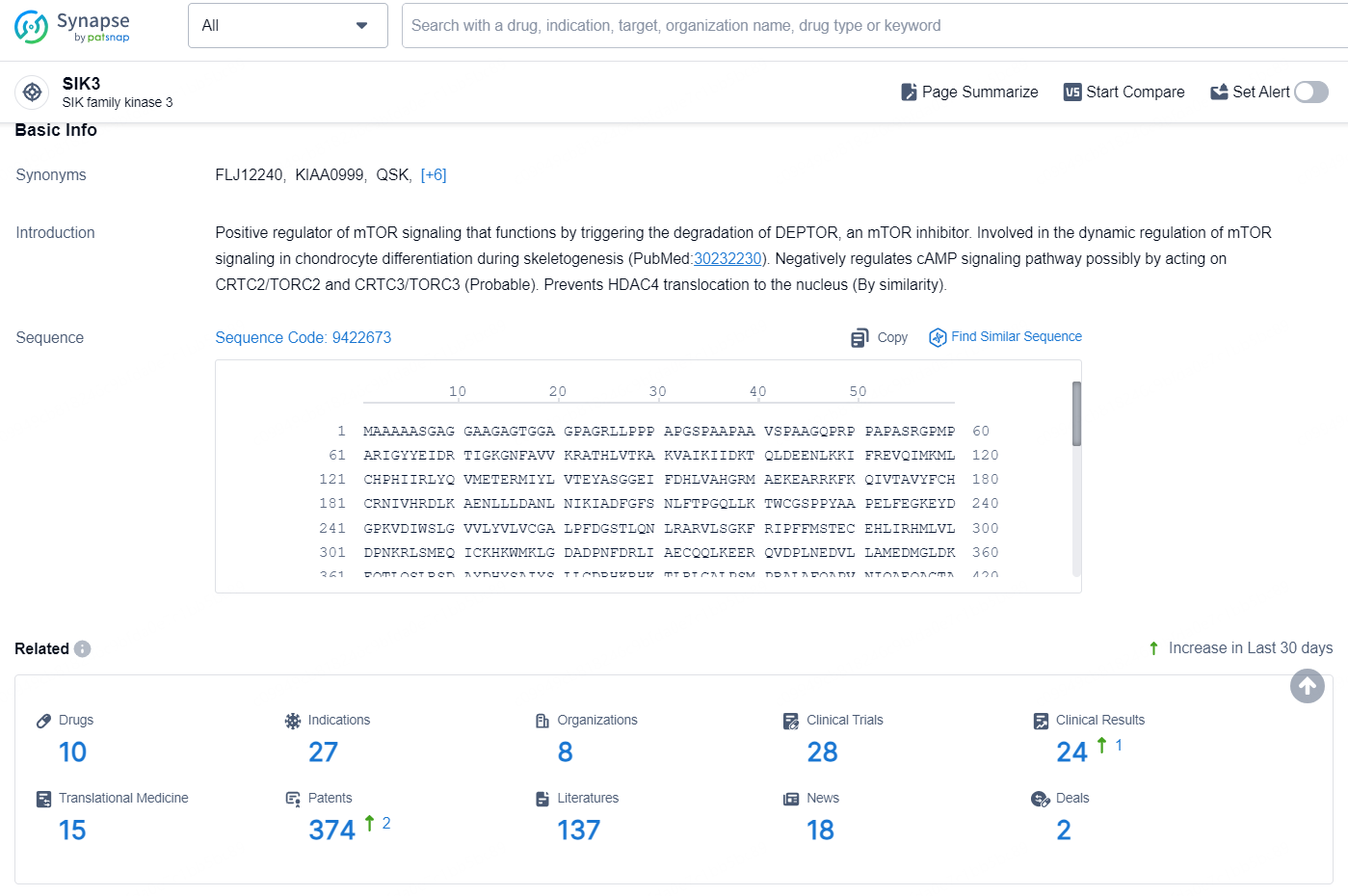
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 26, 2024, there are 10 investigational drugs for the SIK3 targets, including 27 indications, 8 R&D institutions involved, with related clinical trials reaching 28, and as many as 374 patents.
OMX-0407 is an orally available and first-in-class spectrum-selective kinase inhibitor targeting key oncology-relevant tyrosine kinases and salt-inducible kinases (SIKs). OMX-0407 directly interferes with tumor cell proliferation through modulation of tyrosine kinase signaling. Complementary to this direct mode of action, OMX-0407 potentiates tumor cell apoptosis in response to death receptor ligands like tumor necrosis factor, reshaping the tumor micro-environment.