Sutro Biopharma Begins Phase 2 REFRαME-L1 Study on Luvelta for Non-Small Cell Lung Cancer Patients
Sutro Biopharma, Inc. (referred to as Sutro or the Company) (NASDAQ: STRO), a clinical-stage oncology firm innovating in site-specific and novel-format antibody drug conjugates (ADCs), announced that the global Phase 2 trial named REFRαME-L1 for luveltamab tazevibulin (luvelta) in non-small cell lung cancer (NSCLC) patients expressing Folate Receptor-α (FRα) has commenced and is now enrolling participants. Preliminary results from this trial are anticipated in the first half of 2025.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“The launch of REFRαME-L1 marks a significant step in expanding the use of luvelta for a broader patient population with FRα expressing cancers. We have amassed strong preclinical evidence that luvelta may offer a vital new treatment option for NSCLC, supported by its precise engineering, ample therapeutic window, and capability to treat patients with lower FRα expression levels,” stated Anne Borgman, M.D., Sutro’s Chief Medical Officer.
Lung cancer remains the primary cause of cancer-related deaths globally. Over half of these patients receive a metastatic diagnosis, resulting in a 5-year survival rate as low as 8%. Despite the availability of various treatment options, most advanced NSCLC patients eventually develop resistance and face fewer treatment alternatives as their disease advances.
FRα is present in several cancer types, including NSCLC, but has minimal expression in normal tissues. Around 30% of adenocarcinoma NSCLC patients exhibit FRα expression, making FRα an appealing therapeutic target for treating advanced NSCLC and offering these patients a chance for targeted therapy.
REFRαME-L1 is a Phase 2 clinical trial designed to assess the safety and efficacy of luvelta in adults with previously treated advanced or metastatic NSCLC with FRα expression of ≥25% Tumor Proportion Score (TPS). The trial plans to administer doses of 4.3 mg/kg of luvelta every three weeks.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
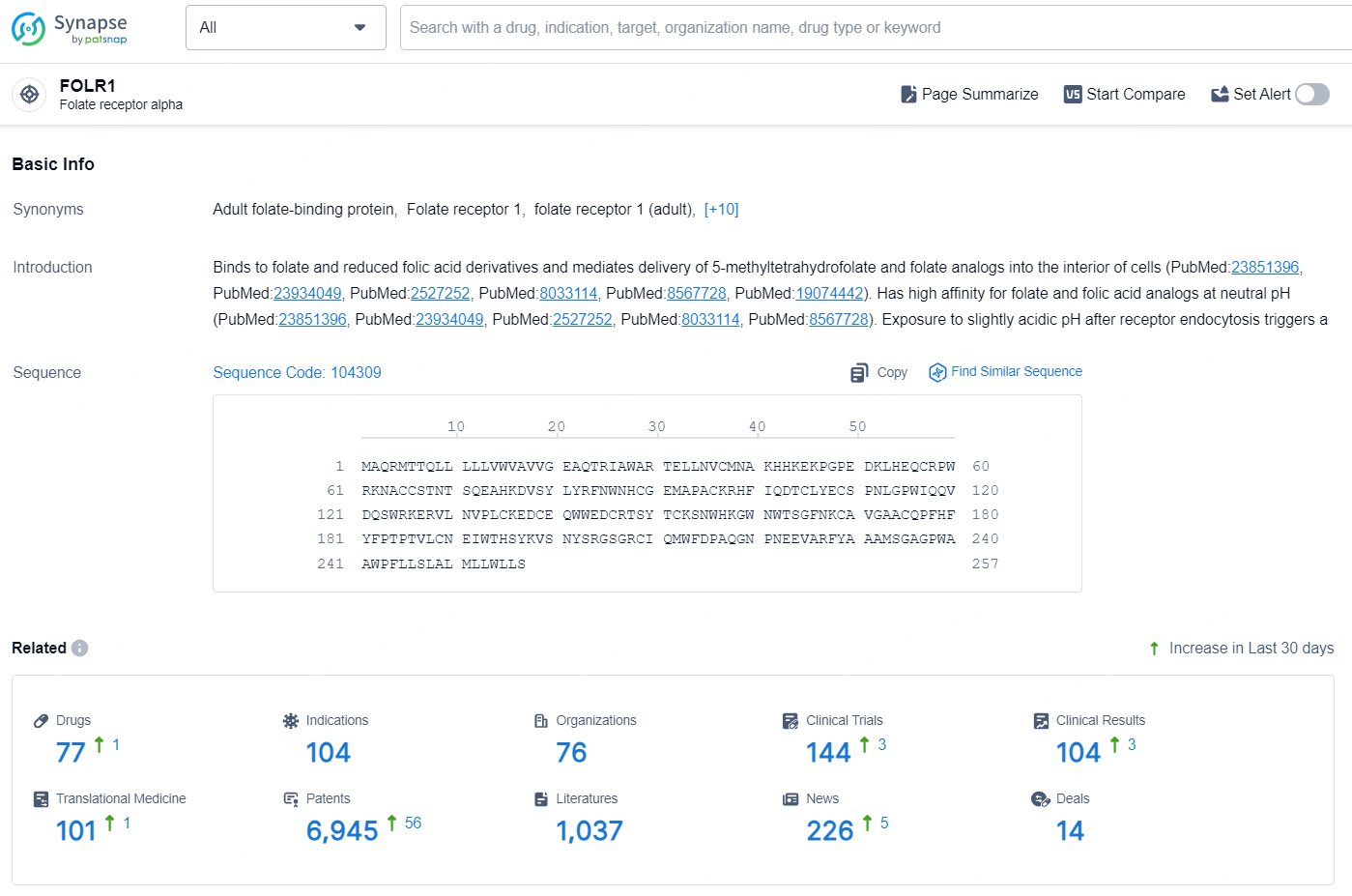
According to the data provided by the Synapse Database, As of August 26, 2024, there are 77 investigational drugs for the FOLR1 target, including 104 indications, 76 R&D institutions involved, with related clinical trials reaching 144, and as many as 6945 patents.
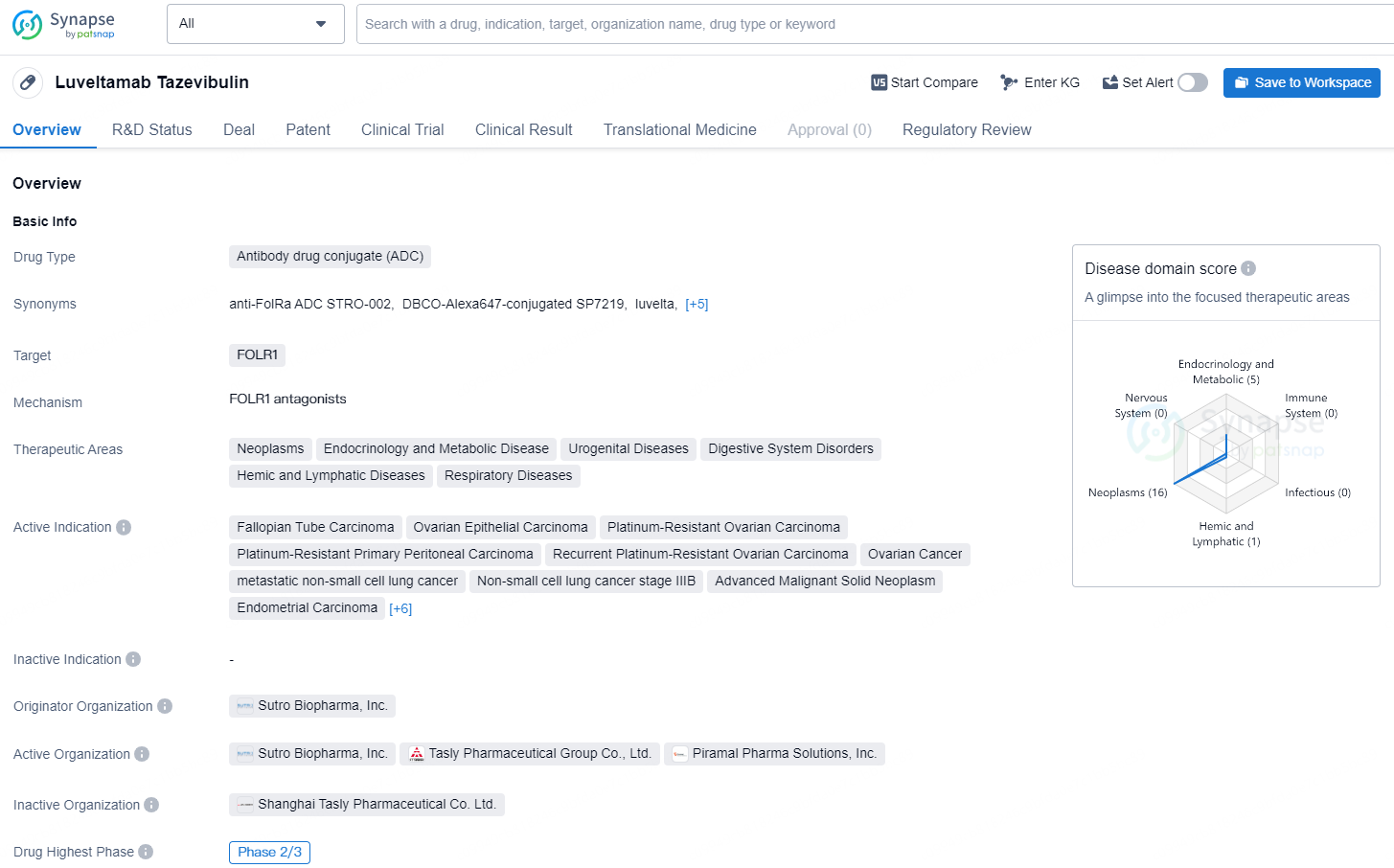
Luveltamab Tazevibulin, an Antibody Drug Conjugate (ADC) designed to target FOLR1, has demonstrated promise in multiple therapeutic domains, including oncology, endocrinology and metabolic disorders, urogenital conditions, gastrointestinal issues, hematologic and lymphatic disorders, as well as respiratory ailments. This drug is being actively pursued for several cancers such as fallopian tube carcinoma, ovarian epithelial carcinoma, platinum-resistant ovarian carcinoma, platinum-resistant primary peritoneal carcinoma, recurrent platinum-resistant ovarian carcinoma, metastatic non-small cell lung cancer, stage IIIB non-small cell lung cancer, advanced malignant solid tumors, endometrial carcinoma, primary peritoneal carcinoma, endometrioid carcinoma, acute myeloid leukemia, adenocarcinoma, non-small cell lung cancer, and various other solid tumors.