Skye Bioscience Begins Phase 2 Trial of CB1 Inhibitor Nimacimab in Obesity Patients
Skye Bioscience, Inc. (Nasdaq: SKYE) (“Skye”), a biopharmaceutical company in its clinical stage committed to discovering new therapeutic pathways for metabolic health, has initiated patient screening for its Phase 2 clinical trial (CBeyondTM) featuring nimacimab, a novel peripheral CB1 inhibitor that acts as a negative allosteric modulating antibody. This study will evaluate the efficacy and safety of nimacimab as an innovative weight loss treatment aimed at reducing weight in obese patients. Parameters that will be considered to determine the long-term quality and sustainability of weight loss include gastrointestinal tolerability (GI) and the preservation of lean body mass.
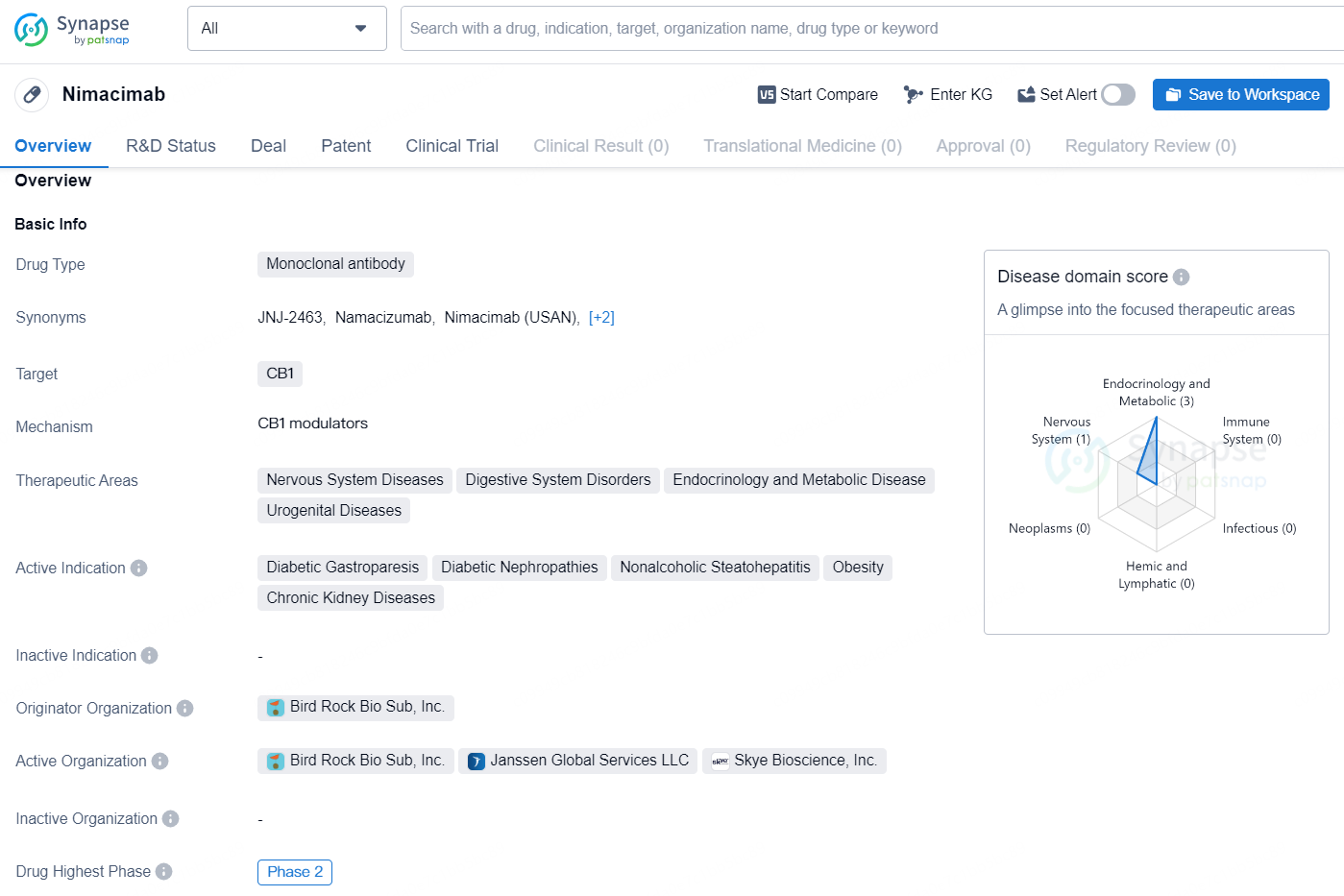
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Clearly, there is an evident requirement for alternative methods of action to provide both physicians and patients with enhanced overall health outcomes in the pursuit of weight loss, beyond what GLP-1 and GIP drugs can achieve. We believe that peripheral CB1 inhibition, and nimacimab—a distinctive biologic drug in this category—possess characteristics that may facilitate such results. We eagerly anticipate reporting interim data from our Phase 2 obesity trial in Q2 2025 and final data in Q4 2025," stated Punit Dhillon, CEO of Skye. "Research into CB1 inhibition has demonstrated its potential to effectively enhance energy expenditure and lipid breakdown1, improve leptin sensitivity2, and modulate hunger and satiety peripherally3. We are gratified to evaluate nimacimab as a standalone treatment and to also conduct a preliminary assessment of our peripheral CB1 inhibitor combined with a GLP-1 drug."
"As we appraise CB1 inhibition as a potential alternative mechanism for weight loss, it is crucial to consider health outcomes beyond gastrointestinal intolerance and muscle loss, such as neuropsychiatric adverse events, which may jeopardize the long-term sustainability of these drugs," added Tu Diep, Chief Development Officer of Skye.
"The first-generation CB1 inhibitor, rimonabant, was a small molecule affecting the CNS. While effective in weight reduction4, it also showed dose-dependent psychiatric adverse events5, including anxiety, depressed mood, depression, and suicidality. Current second-generation CB1 inhibitors, small molecules potentially more peripherally restricted than rimonabant, still exhibit brain accumulation6 in preclinical models, indicating a possible persistent safety issue. Nimacimab, a monoclonal antibody (large molecule), is significantly more brain-restricted7, with no psychiatric adverse events observed in our preclinical studies7 or Phase 1 study7 in nonalcoholic fatty liver disease (NAFLD) patients. We believe nimacimab’s favorable safety and tolerability give Skye a competitive edge over small molecule CB1 inhibitors."
"We are deeply honored to engage in this study of nimacimab as an adjunct therapy for obesity treatment. This novel strategy targeting the CB1 receptor is a logical advancement following the promising outcomes in early trials," commented Harold Edward Bays, MD, Medical Director/President of Louisville Metabolic and Atherosclerosis Research Center/Your Body Goal. "We hope that investigating this alternative mechanism of action will ultimately expand the therapeutic options available for patients dealing with obesity."
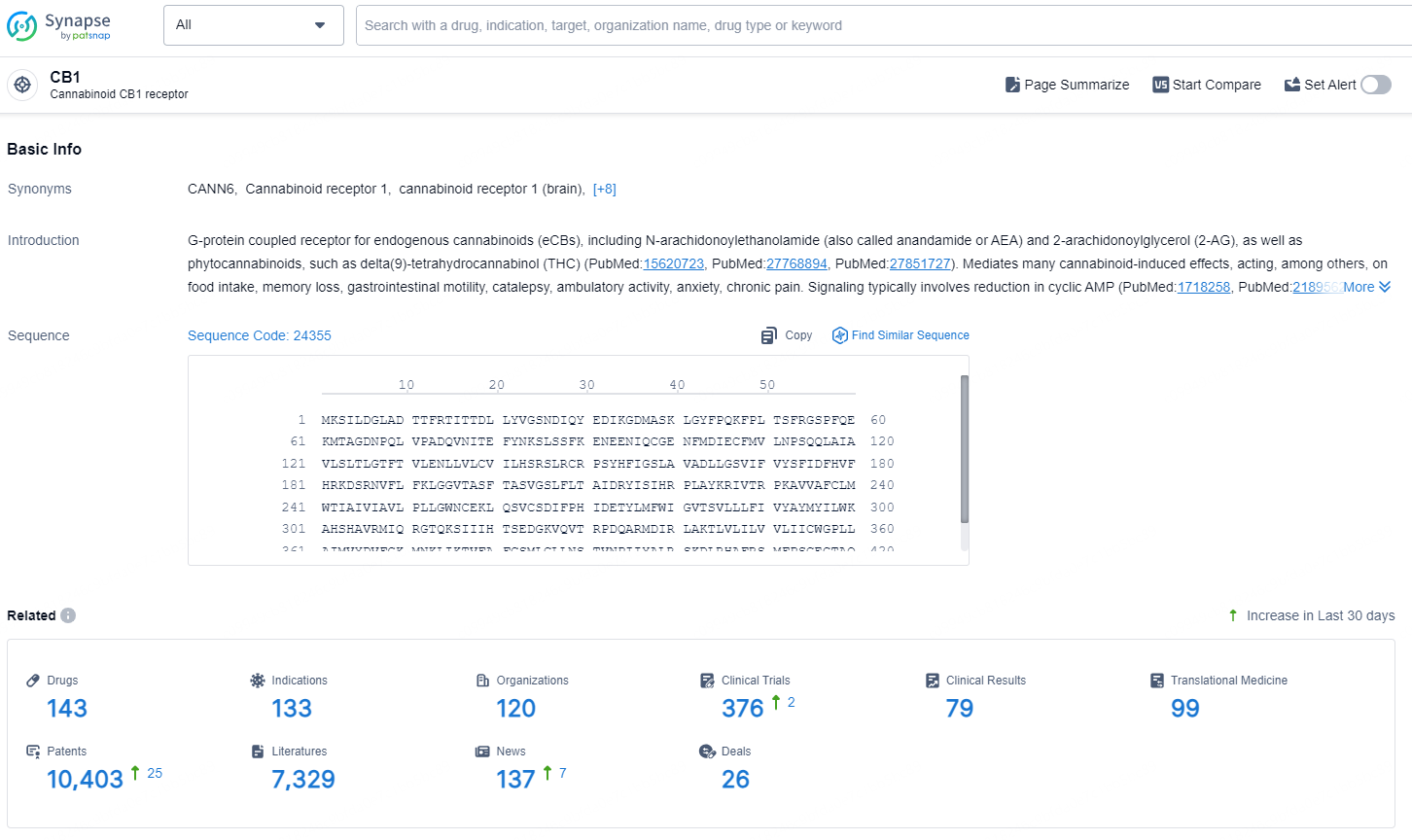
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 26, 2024, there are 143 investigational drugs for the CB1 targets, including 133 indications, 120 R&D institutions involved, Nimacimab is a monoclonal antibody that targets the CB1 receptor and is being developed for therapeutic areas including Nervous System Diseases, Digestive System Disorders, Endocrinology and Metabolic Disease, and Urogenital Diseases. Its active indications are for Diabetic Gastroparesis, Diabetic Nephropathies, Nonalcoholic Steatohepatitis, Obesity, and Chronic Kidney Diseases. The drug is currently in Phase 2 of development and is being developed by Bird Rock Bio Sub, Inc.