Is Vutrisiran approved by the FDA?
Vutrisiran received FDA approval on June 13, 2022, under the brand name Amvuttra. It is an FDA-approved medication used to treat polyneuropathy in adults with hereditary transthyretin-mediated amyloidosis (hATTR). This condition arises from faulty transthyretin (TTR) proteins produced by the liver, leading to the accumulation of TTR deposits in various organs, predominantly affecting peripheral nerves.
Mechanism of Action: Vutrisiran is an injectable small interfering RNA (siRNA) – GalNAc conjugate. It works by targeting liver hepatocytes, where it binds to both variant and wild-type TTR messenger RNA (mRNA). This process results in the degradation of these mRNAs, thereby reducing the production of TTR protein and amyloid deposits in tissues. This mechanism helps alleviate symptoms of polyneuropathy associated with hATTR.
Dosage and Administration: The recommended dosage of vutrisiran is 25 mg, administered subcutaneously in the abdomen (excluding within 2 inches of the belly button), upper thighs, or the back of the upper arms. It is available as a prefilled syringe ready for administration.
Side Effects: Common side effects of vutrisiran include pain in the extremities (hands and feet), joint pain, shortness of breath, and low levels of vitamin A. Mild and transient injection site reactions are also reported in some patients. Serious side effects may include vision problems related to vitamin A deficiency, requiring careful monitoring and appropriate vitamin A supplementation as prescribed by healthcare providers.
Precautions: Before starting vutrisiran, inform your healthcare provider about any existing medical conditions, allergies, pregnancy, or plans for breastfeeding. Vutrisiran may harm unborn babies and should not be used during pregnancy unless clearly necessary. It is advised to avoid breastfeeding while on vutrisiran treatment due to potential risks to the infant.
Drug Interactions: While no formal clinical drug interaction studies have been conducted, vutrisiran is not expected to interact significantly with other medications or affect cytochrome P450 enzyme activity.
Storage: Store vutrisiran in the refrigerator between 2°C to 30°C (36°F to 86°F) in its original carton. Do not freeze the medication.
Conclusion: In conclusion, vutrisiran (Amvuttra) represents a significant advancement in the treatment of polyneuropathy associated with hATTR, offering a targeted therapeutic approach through its innovative siRNA technology. Its FDA approval in 2022 underscores its efficacy and safety profile in managing this debilitating genetic condition.
How to obtain the latest development progress of all drugs?
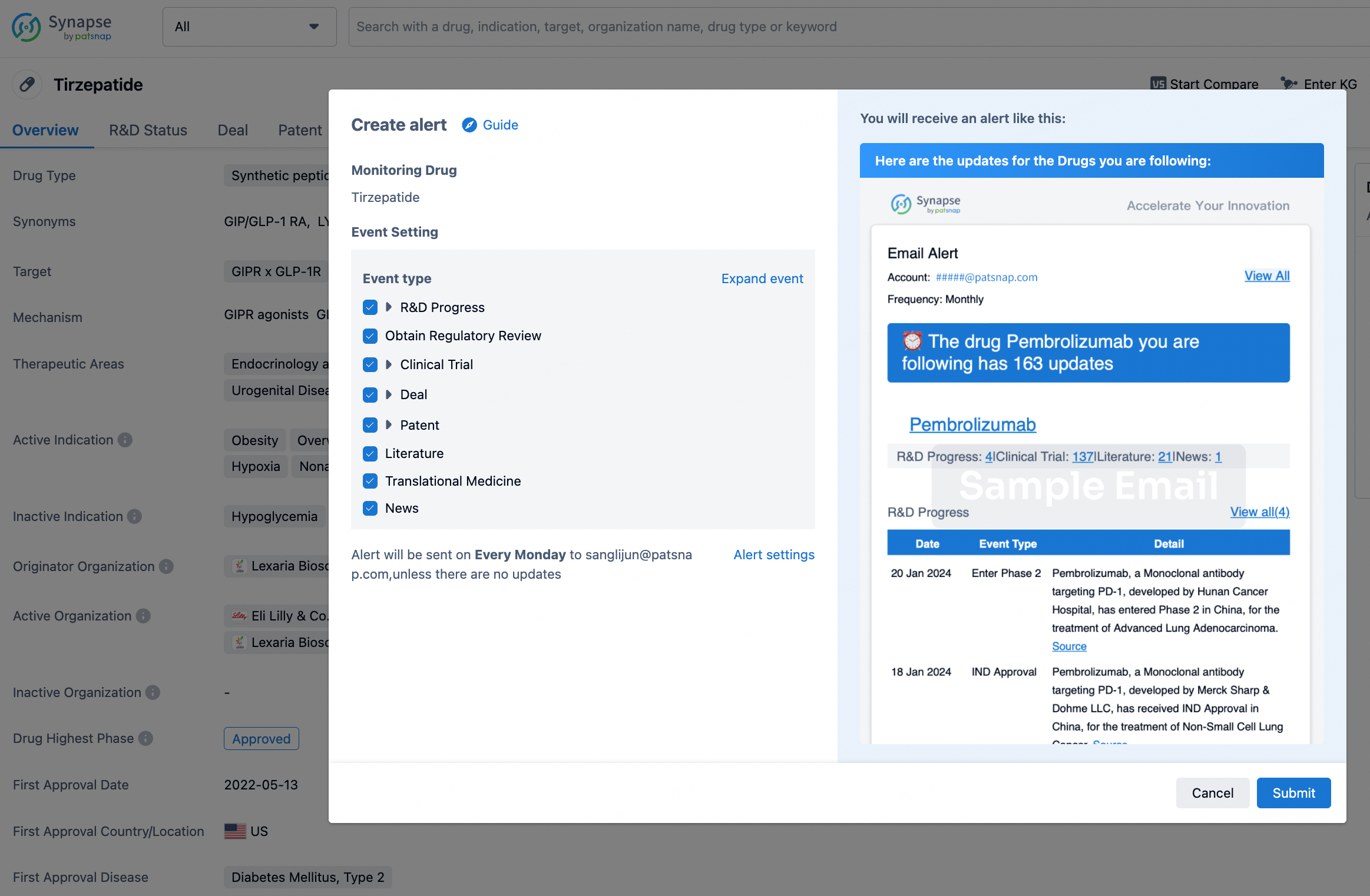
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!