Is Berotralstat approved by the FDA?
Berotralstat, marketed under the brand name Orladeyo, is a medication designed to prevent attacks of hereditary angioedema (HAE) in adults and children aged 12 years and older. Berotralstat received approval from the US Food and Drug Administration (FDA) on December 3, 2020. The approval was granted for the prevention of HAE attacks, making it a significant development for individuals suffering from this rare genetic condition.This condition is characterized by recurrent episodes of severe swelling in various parts of the body, including the extremities, face, gastrointestinal tract, and airways.
Uses and Administration
Berotralstat is specifically indicated for preventing HAE attacks and is not intended for treating an acute attack that has already begun. The medication is taken orally once daily with food. It is crucial to follow the prescribed dosage instructions closely, as exceeding the recommended dose can lead to serious heart rhythm problems, such as QT prolongation.
Dosage:
- Adults and Children (12 years and older): 150 mg orally once daily with food.
- The medication should be stored at room temperature and kept in its blister pack until ready to use.
Side Effects
Common Side Effects:
- Stomach pain, heartburn, vomiting
- Diarrhea
- Back pain
Serious Side Effects:
- Allergic reactions: hives, difficulty breathing, swelling of the face, lips, tongue, or throat
Patients should immediately seek medical help if they experience any signs of an allergic reaction. Regular communication with a healthcare provider is essential for managing any side effects and ensuring safe use of the medication.
Warnings and Precautions
- Medical Conditions: Inform your doctor if you have liver disease, kidney disease, or heart rhythm problems.
- Pregnancy and Breastfeeding: Discuss with your doctor if you are pregnant, planning to become pregnant, or breastfeeding.
- Age Restriction: Not approved for use in individuals younger than 12 years old.
Drug Interactions
Berotralstat can interact with other medications, including prescription drugs, over-the-counter medicines, vitamins, and herbal products. It is essential to inform your healthcare provider about all the medications you are currently taking to avoid potential interactions.
Conclusion
This medication offers a significant therapeutic option for individuals managing this rare genetic disorder. Patients must adhere to the prescribed dosage and report any side effects to their healthcare provider to ensure the safe and effective use of Berotralstat.
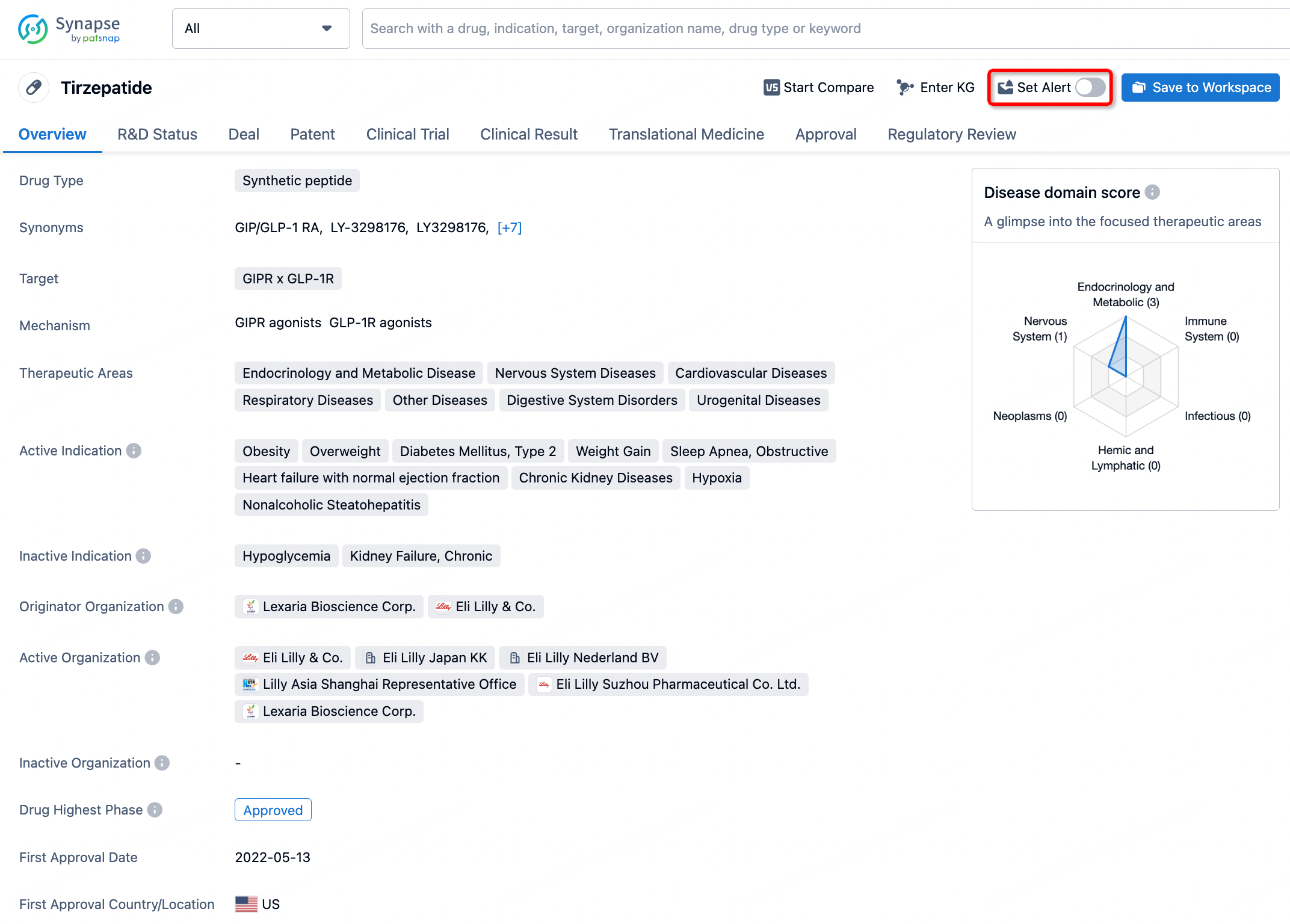
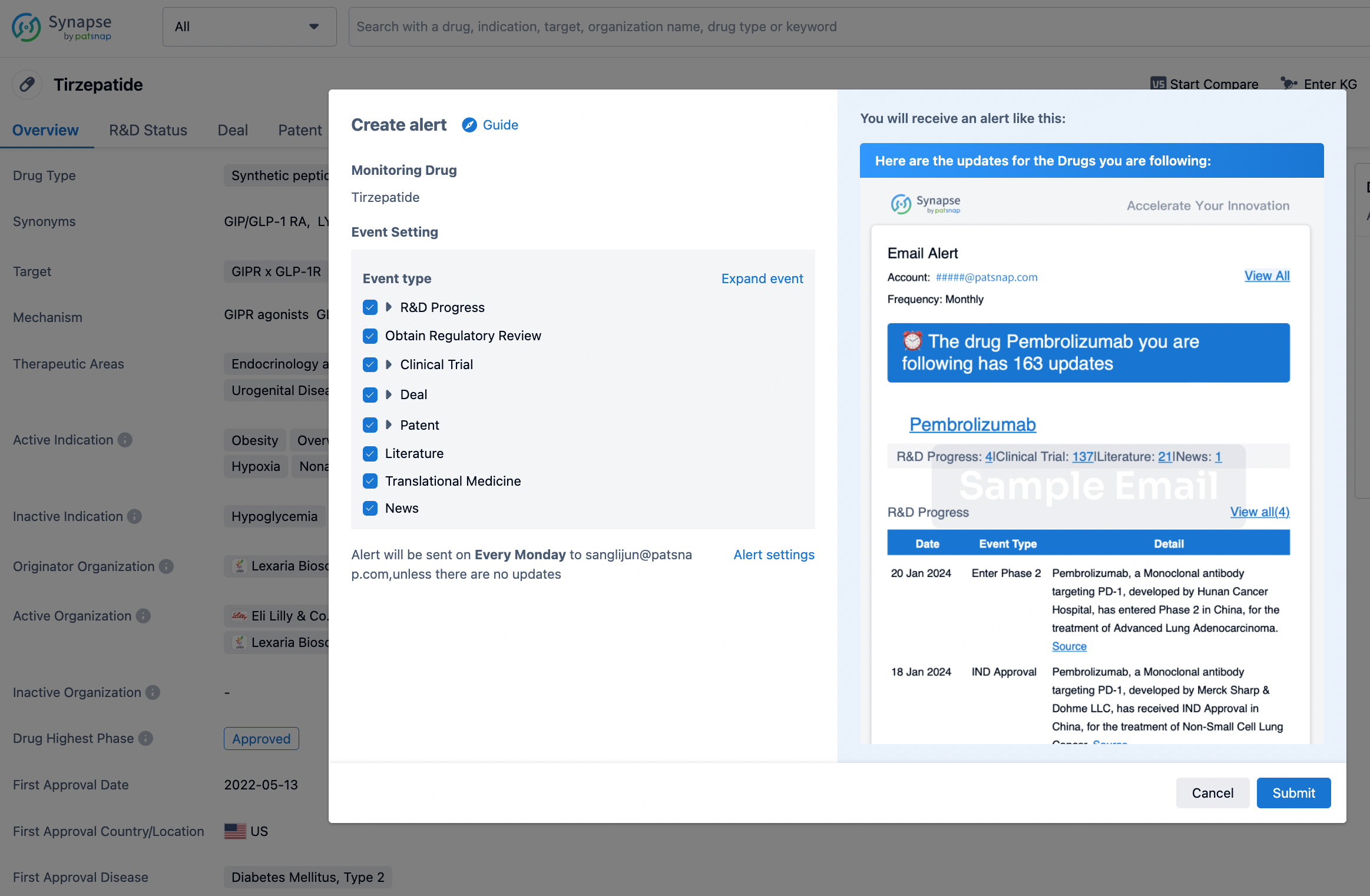
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!