Is Elranatamab approved by the FDA?
Yes, Elranatamab, marketed under the brand name Elrexfio, is FDA approved. It received FDA approval for the treatment of adults with multiple myeloma who have undergone at least four prior treatment regimens, and whose cancer has either returned or did not respond to previous treatments.
Uses
Elranatamab is used to treat adults with multiple myeloma, a type of blood cancer. It is particularly prescribed for patients who have tried at least four other treatment regimens without success or whose cancer has relapsed.
Mechanism of Action
Elranatamab belongs to the miscellaneous antineoplastics drug class, which includes a variety of cancer-fighting medications with different mechanisms of action. Its specific mechanism involves targeting and destroying cancerous cells in patients with multiple myeloma, providing a new option for those with difficult-to-treat forms of this disease.
Dosage and Administration
- Administration: Elranatamab is administered by a healthcare provider as a subcutaneous injection, typically in the abdomen, though other areas may be used.
- Monitoring: Patients receiving Elranatamab may need to stay in the hospital for 24 to 48 hours after some doses to monitor for serious side effects or allergic reactions. Frequent medical tests are necessary before and during treatment to ensure safety and efficacy.
Side Effects
Common side effects include:
- Bone pain, muscle pain
- Diarrhea, nausea
- Fever, rash, cough
- Loss of appetite
- Pain, bruising, swelling, or irritation at the injection site
- Tiredness
Serious side effects include:
- Signs of cytokine release syndrome (CRS): fever, chills, trouble breathing, confusion, severe vomiting or diarrhea, irregular heartbeats, feeling light-headed or very tired.
- Infections: cold symptoms, stuffy nose, sneezing, sore throat, fever, night sweats, swollen glands, cold sores, cough, wheezing, diarrhea, chest pain, pain in the side or lower back, painful urination, blood or pus in the urine.
- Liver problems: loss of appetite, nausea, vomiting, stomach pain (upper right side), tiredness, itching, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes).
- Low white blood cell counts: fever, mouth sores, skin sores, sore throat, cough.
- Neurological problems: headache, jerking muscle movements, rigid muscles, restlessness, numbness and tingling, confusion, problems speaking, muscle spasms, tremors, double vision, changes in handwriting, problems walking, muscle weakness, hearing loss, burning, throbbing, or stabbing pain.
Patients should report any side effects to their doctor and can also report them to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Neurological Symptoms: Patients should immediately report symptoms such as headache, seizures, confusion, problems with thinking or memory, muscle weakness, numbness and tingling, tremor, problems with speech or walking.
- Pregnancy and Breastfeeding: Elranatamab can harm an unborn baby. Women should use effective birth control during treatment and for at least 4 months after the last dose. Breastfeeding is not recommended during treatment and for at least 4 months after the last dose.
- Infections and Liver Function: Patients with low white blood cell counts, active or recent infections, or abnormal liver function tests should inform their doctor before starting treatment.
Additional Information
Elranatamab is available only through a special program and must be obtained from a certified pharmacy. Patients must be registered in this program to receive the medication. A Patient Wallet Card, which includes information about serious side effects and symptoms to watch for, should be kept by the patient at all times.
Summary
Elranatamab (Elrexfio) is FDA approved for the treatment of adults with multiple myeloma who have received at least four prior treatment regimens and whose cancer has relapsed or did not respond to previous treatments. Administered as a subcutaneous injection, Elranatamab requires careful monitoring due to its potential for serious side effects, including cytokine release syndrome, infections, liver problems, and neurological issues. Its approval provides a vital option for patients with limited treatment choices in their battle against multiple myeloma.
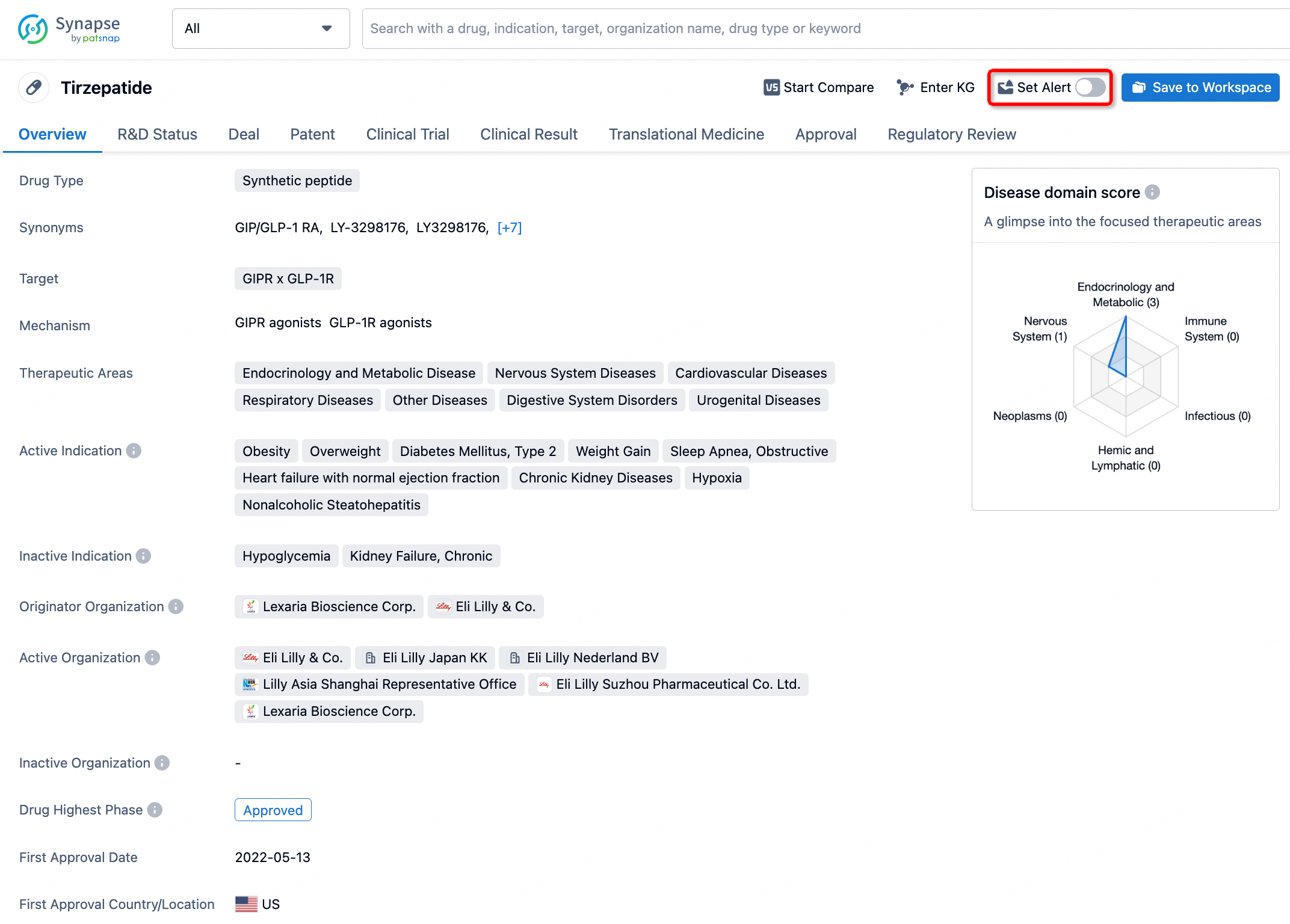
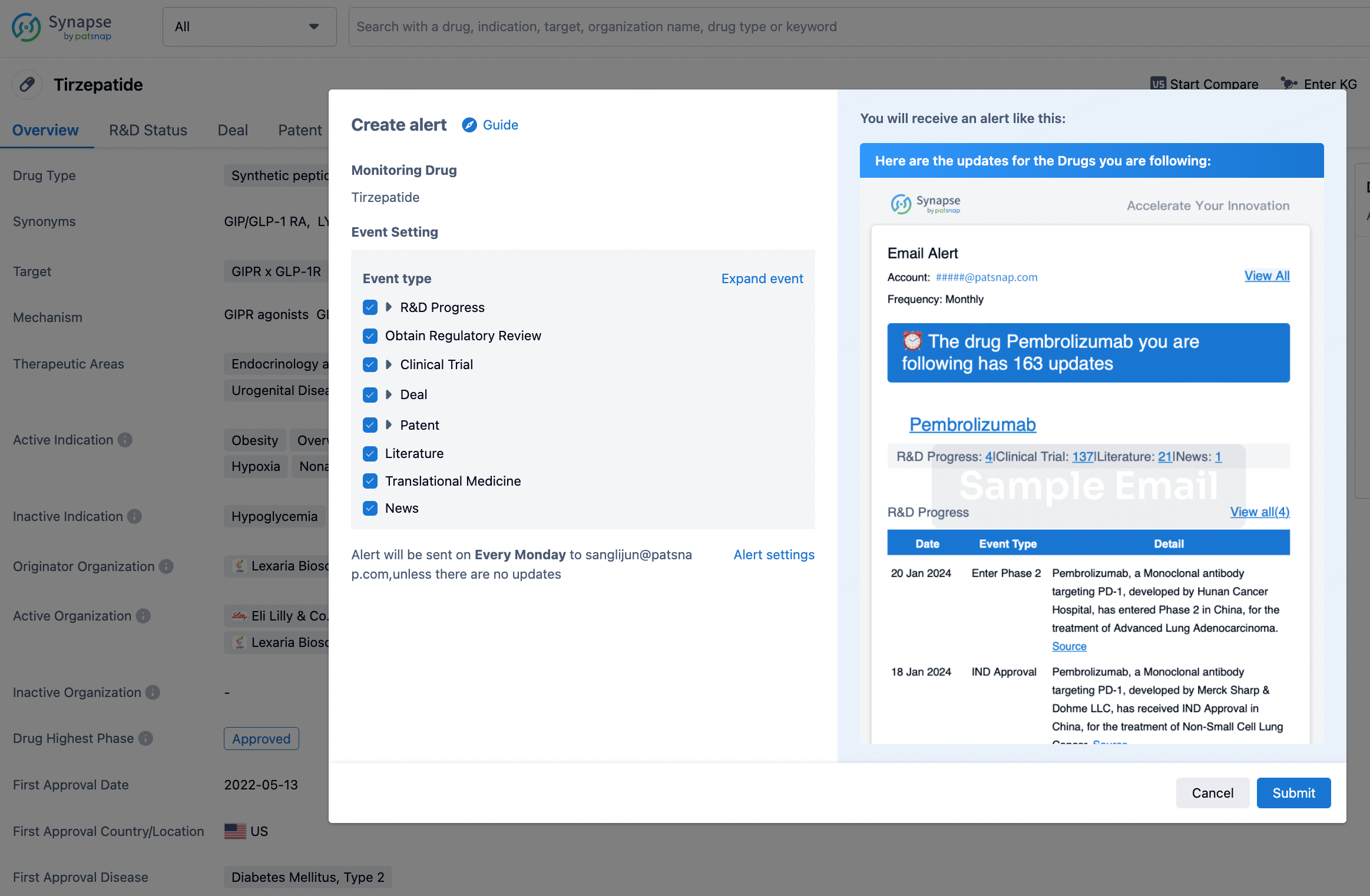
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!