Is Etranacogene dezaparvovec approved by the FDA?
Etranacogene dezaparvovec, marketed under the brand name Hemgenix, is a gene therapy used to treat adults with Hemophilia B. Etranacogene dezaparvovec received FDA approval on November 22, 2022. This condition is a genetic bleeding disorder caused by insufficient levels of Factor IX, a protein necessary for blood clotting. Etranacogene dezaparvovec is specifically used for patients who are on preventive treatment, have recurrent serious bleeding episodes, or have experienced life-threatening bleeding events.
How It Works
Etranacogene dezaparvovec is administered as a single intravenous infusion. The therapy works by delivering a functional copy of the Factor IX gene into the patient’s liver cells, enabling the production of the missing protein. This approach aims to provide long-term benefits by potentially reducing or eliminating the need for regular Factor IX infusions.
Side Effects and Warnings
Common side effects of etranacogene dezaparvovec include:
- Abnormal blood tests
- Headache
- Flu-like symptoms
- Reactions at the injection site
- Fatigue and body aches
Some patients may experience serious side effects, particularly during or immediately after the infusion, such as dizziness, nausea, light-headedness, itching, sweating, headache, chest tightness, back pain, trouble breathing, unusual bleeding, or swelling in the face. It is crucial to monitor patients closely during and for at least three hours after the infusion to manage any adverse reactions promptly.
Precautions
Before starting treatment, patients undergo a series of medical tests to ensure suitability for etranacogene dezaparvovec therapy. These tests include screening for Factor IX inhibitors and assessing liver health through enzyme testing, hepatic ultrasound, and elastography. Regular follow-up tests, especially liver function tests, are necessary for three months post-treatment to monitor for potential liver-related side effects.
Contraindications and Interactions
Patients with a positive Factor IX inhibitor test, significant liver abnormalities, or chronic active infections should not receive etranacogene dezaparvovec. The therapy is not intended for use in women and may interact with various medications, including prescription and over-the-counter drugs, vitamins, and herbal products. It is essential to inform healthcare providers of all medications being taken to avoid adverse interactions.
In summary, etranacogene dezaparvovec (Hemgenix) is FDA-approved for the treatment of Hemophilia B in adults, offering a promising gene therapy approach to managing this bleeding disorder. Patients considering this treatment should work closely with their healthcare providers to understand the potential benefits and risks associated with the therapy.
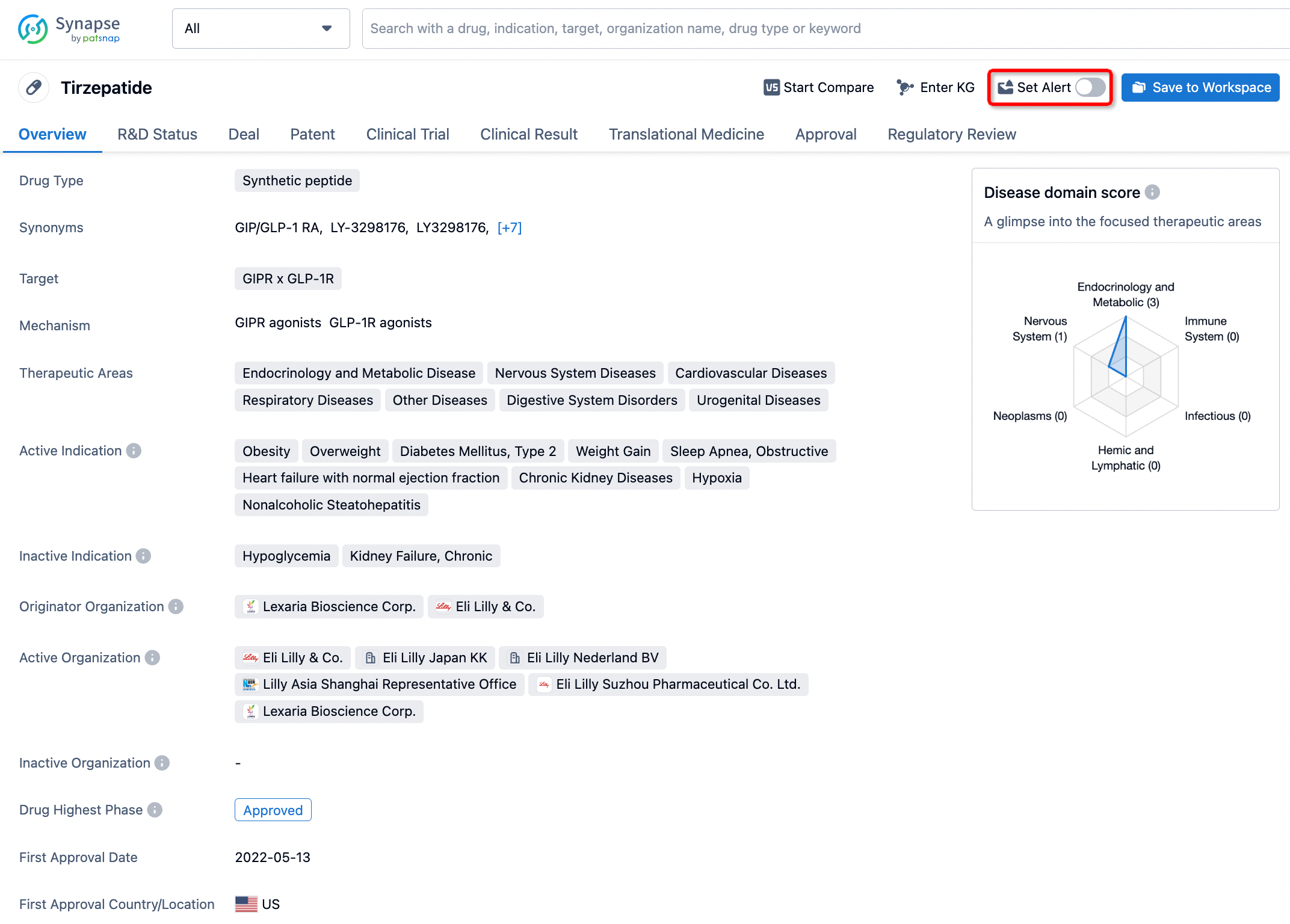
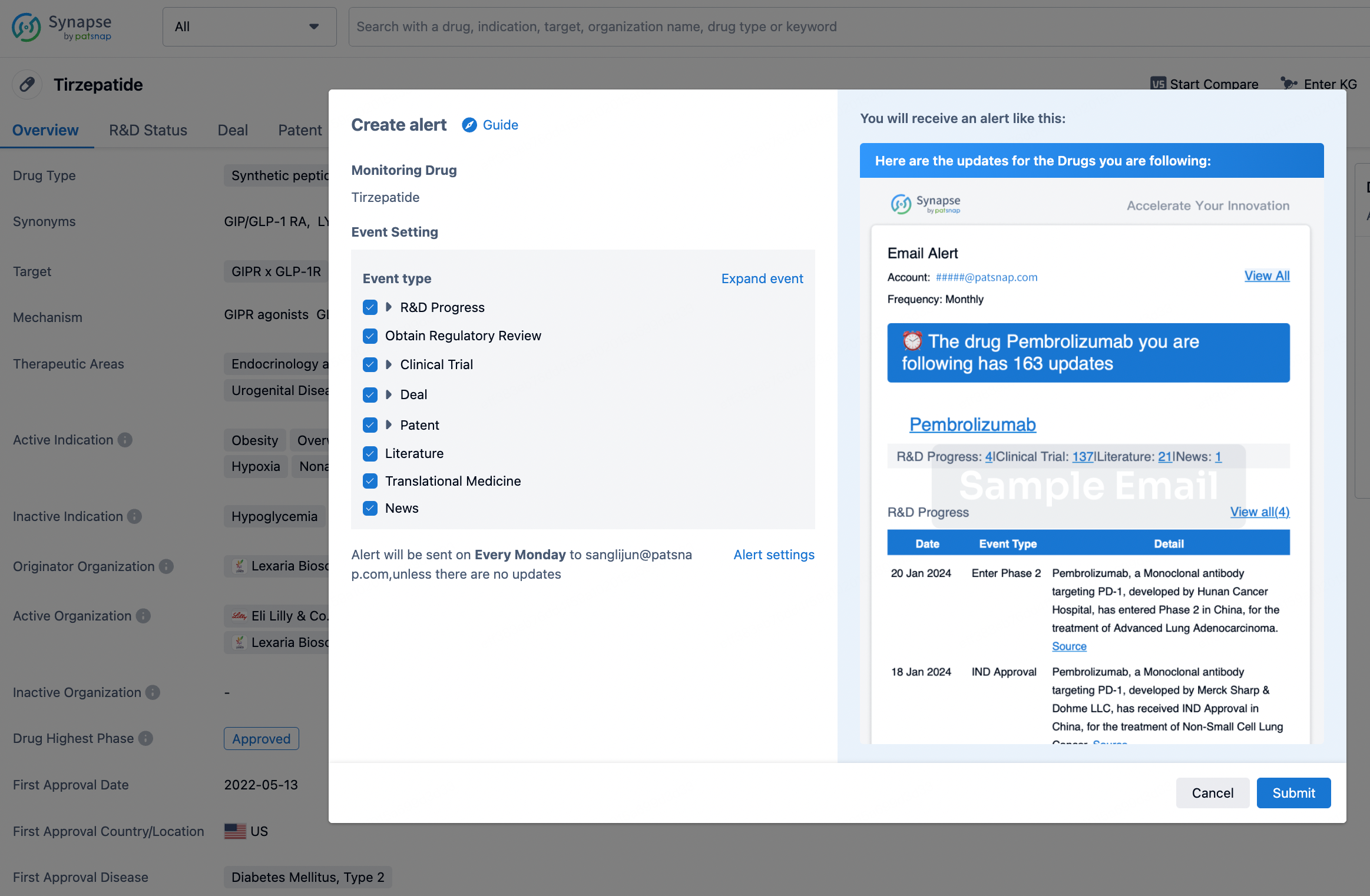
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!