Is Voclosporin approved by the FDA?
Voclosporin, marketed under the brand name Lupkynis, is an oral medication that belongs to the drug class of calcineurin inhibitors. Voclosporin was approved by the U.S. Food and Drug Administration (FDA) on January 22, 2021. This approval marked a significant advancement in the treatment of lupus nephritis, offering a new therapeutic option for patients suffering from this severe condition.
Uses and Administration
Uses:
- Voclosporin is prescribed to treat adults with active lupus nephritis in combination with background immunosuppressive therapy such as mycophenolate mofetil (MMF) and corticosteroids.
- The primary goal is to reduce kidney inflammation and prevent further kidney damage.
Administration:
- The usual adult dose is 23.7 mg taken orally twice a day.
- The medication should be taken on an empty stomach, at least one hour before or two hours after a meal.
- Doses should be taken at regular intervals 12 hours apart, and missed doses should be skipped if more than four hours late.
- Patients should swallow the capsule whole without crushing, chewing, breaking, or opening it.
Precautions and Considerations
Before Taking Voclosporin:
- Inform your doctor of any allergies or adverse reactions to medications.
- Discuss all other medications, including over-the-counter drugs, vitamins, and herbal supplements, with your healthcare provider to avoid potential interactions.
- Do not use voclosporin if you are pregnant or planning to become pregnant, as it contains alcohol and can harm the unborn baby. Use effective birth control during treatment and for at least seven days after the last dose.
- Breastfeeding is not recommended during treatment with voclosporin.
Potential Interactions:
- Voclosporin should not be used in combination with cyclophosphamide, nefazodone, certain antibiotics (e.g., clarithromycin, telithromycin), antifungal medicines (e.g., itraconazole, ketoconazole), or certain antiviral medications used to treat HIV or hepatitis C (e.g., boceprevir, cobicistat).
- Avoid grapefruit products as they may lead to unwanted side effects.
Side Effects
Common Side Effects:
- Kidney problems
- Anemia
- High blood pressure
- Stomach pain, heartburn, loss of appetite, diarrhea
- Tremors
- Mouth sores
- Headache, tiredness
- Painful urination
- Cough
- Hair loss
Serious Side Effects:
- Signs of an allergic reaction: hives, difficult breathing, swelling of the face, lips, tongue, or throat
- Pain or burning during urination
- Kidney problems: little or no urination, swelling in the feet or ankles, feeling tired or short of breath
- High blood pressure: severe headache, blurred vision, pounding in the neck or ears
- Nervous system problems: confusion, vision changes, headache, feeling less alert, tremors, numbness, tingling, seizure
- High potassium level: nausea, weakness, chest pain, irregular heartbeats
- Low red blood cells (anemia): pale skin, unusual tiredness, light-headedness, shortness of breath, cold hands and feet
Patients experiencing serious side effects should seek immediate medical attention. For any concerns about side effects, contacting a healthcare provider is recommended, and adverse effects can be reported to the FDA at 1-800-FDA-1088.
Conclusion
It offers an important treatment option to help manage this serious kidney condition in patients with systemic lupus erythematosus. As with any medication, patients should follow their healthcare provider's instructions and be aware of potential side effects and interactions with other medications.
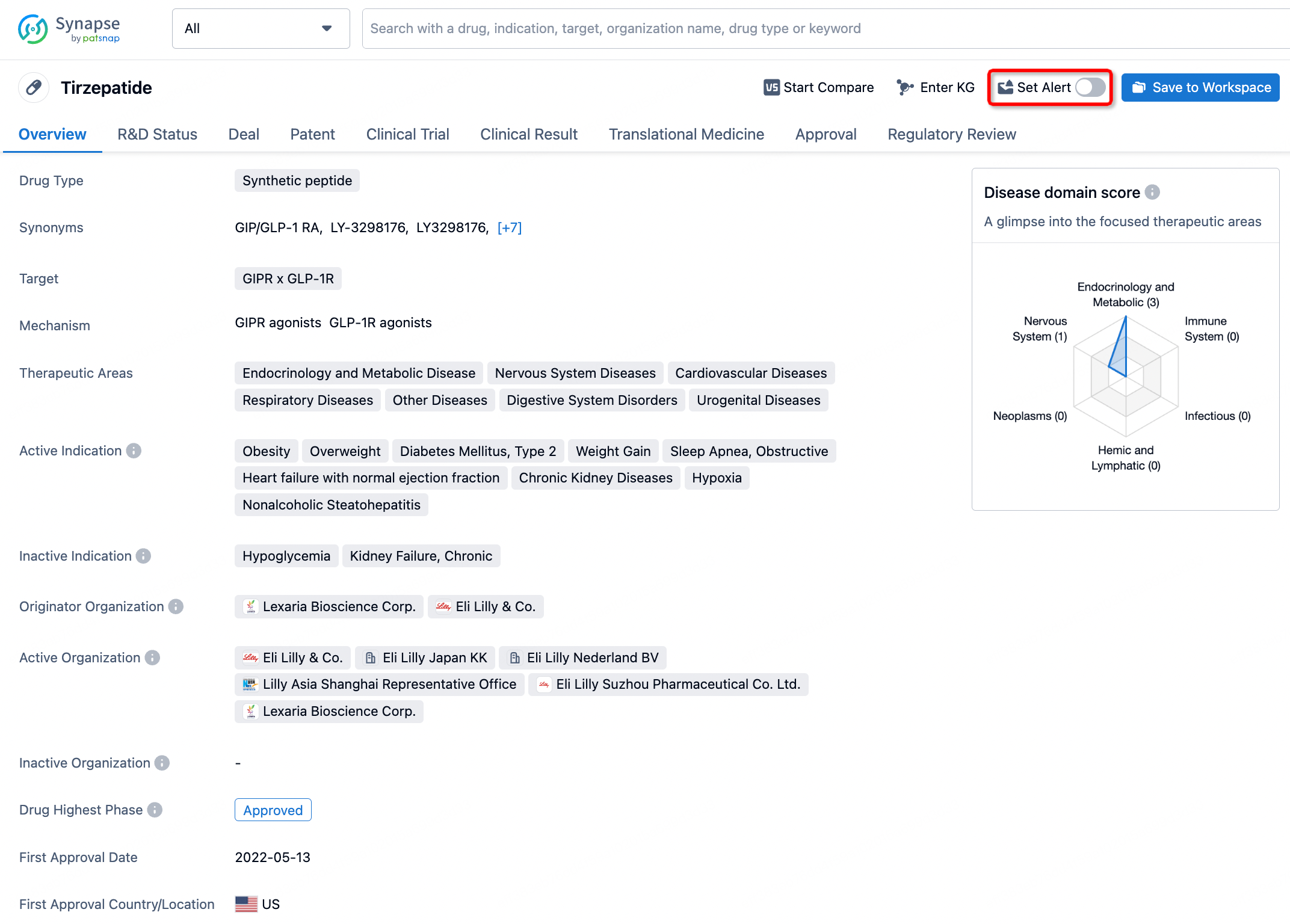
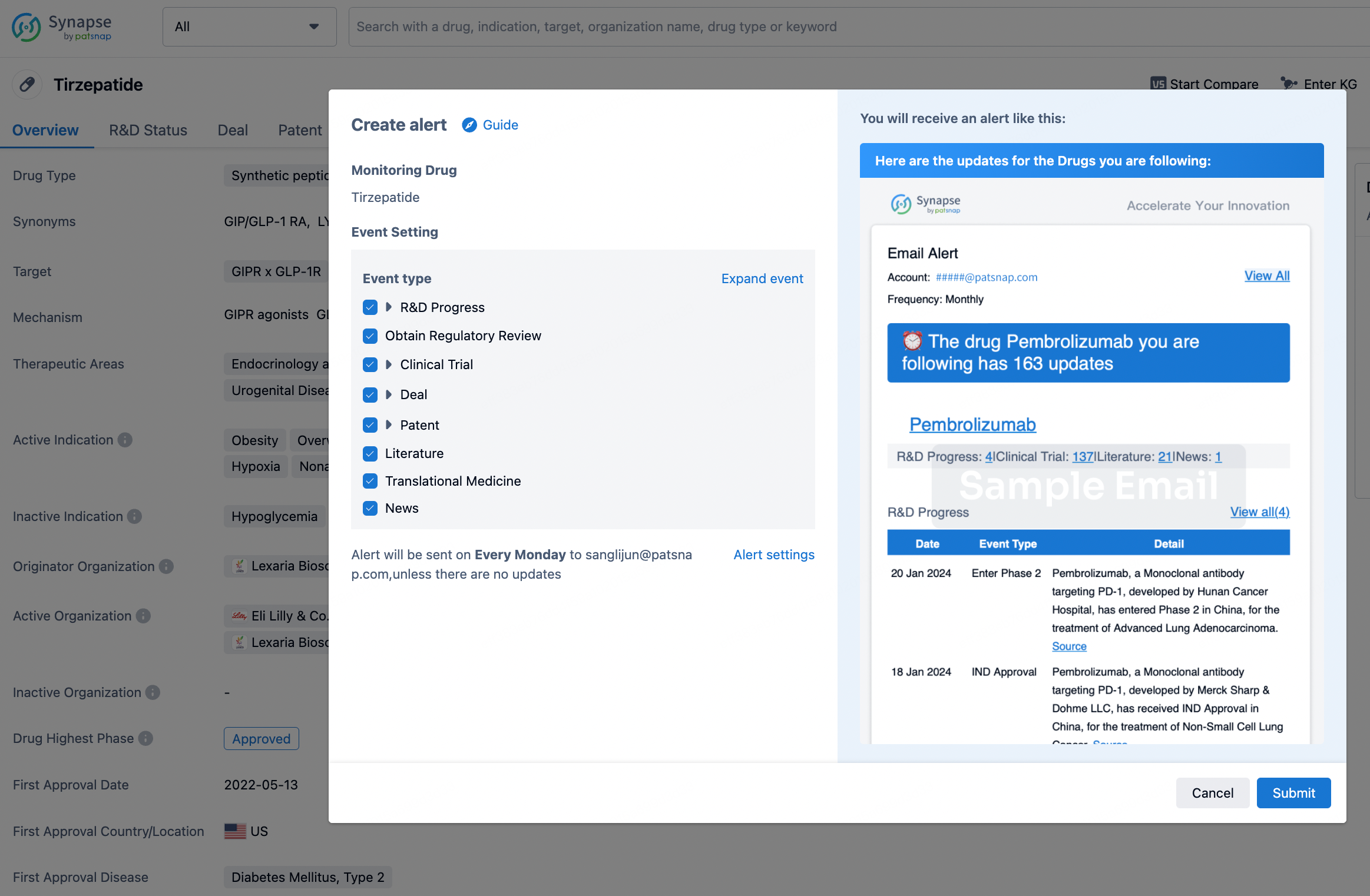
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!