Is Idecabtagene vicleucel approved by the FDA?
Idecabtagene vicleucel, commonly known by its brand name Abecma, is a pioneering immunotherapy used in the treatment of multiple myeloma, a type of blood cancer. Abecma (idecabtagene vicleucel) received approval from the U.S. Food and Drug Administration (FDA) on March 26, 2021. This marked a significant advancement in cancer treatment, providing a new option for patients with relapsed or refractory multiple myeloma who have exhausted other treatments.
Mechanism of Action
Abecma is a type of CAR-T cell therapy. It is created using the patient’s own white blood cells, which are collected and genetically modified to express a receptor that targets and kills multiple myeloma cells. This personalized treatment involves several steps:
- Leukapheresis: Collection of white blood cells from the patient.
- Genetic Modification: The cells are engineered in a lab to produce chimeric antigen receptors (CARs).
- Expansion: The modified cells are multiplied.
- Infusion: After preparatory chemotherapy, the CAR-T cells are infused back into the patient.
Usage and Administration
Idecabtagene vicleucel is administered in a specialized medical setting due to its complexity and the potential for severe side effects. The process includes:
- Pre-treatment with Chemotherapy: Given to prepare the body for the CAR-T cell infusion.
- Infusion: The modified cells are infused intravenously.
- Monitoring: Patients are closely monitored for at least seven days post-infusion to manage any immediate adverse reactions.
Side Effects and Warnings
Common Side Effects:
- Cytokine release syndrome (CRS): Symptoms include fever, chills, difficulty breathing, and rapid heartbeats.
- Neurological toxicities: Issues like confusion, memory problems, and seizures.
- Infections: Due to immunosuppression.
- Low blood cell counts: Leading to symptoms like fatigue, increased infection risk, and easy bruising.
Severe Side Effects:
- CRS: This is a potentially life-threatening condition that requires immediate medical intervention.
- Neurological toxicities: Severe effects such as encephalopathy and seizures necessitate close monitoring.
Patients must remain near the treatment facility for at least four weeks after infusion to ensure any severe side effects can be promptly managed.
Precautions
Before starting idecabtagene vicleucel, it’s essential to inform the healthcare provider about:
- Any active infections.
- History of hepatitis B or cytomegalovirus.
- Recent vaccinations (within the last six weeks).
Pregnancy and Breastfeeding:
- Women may need to undergo pregnancy testing and use effective contraception during and shortly after treatment.
- It may not be safe to breastfeed during treatment.
Lifestyle Considerations:
- Avoid driving or operating heavy machinery for at least eight weeks post-treatment due to potential cognitive and motor impairments.
- Refrain from receiving live vaccines and donating blood, organs, or tissues.
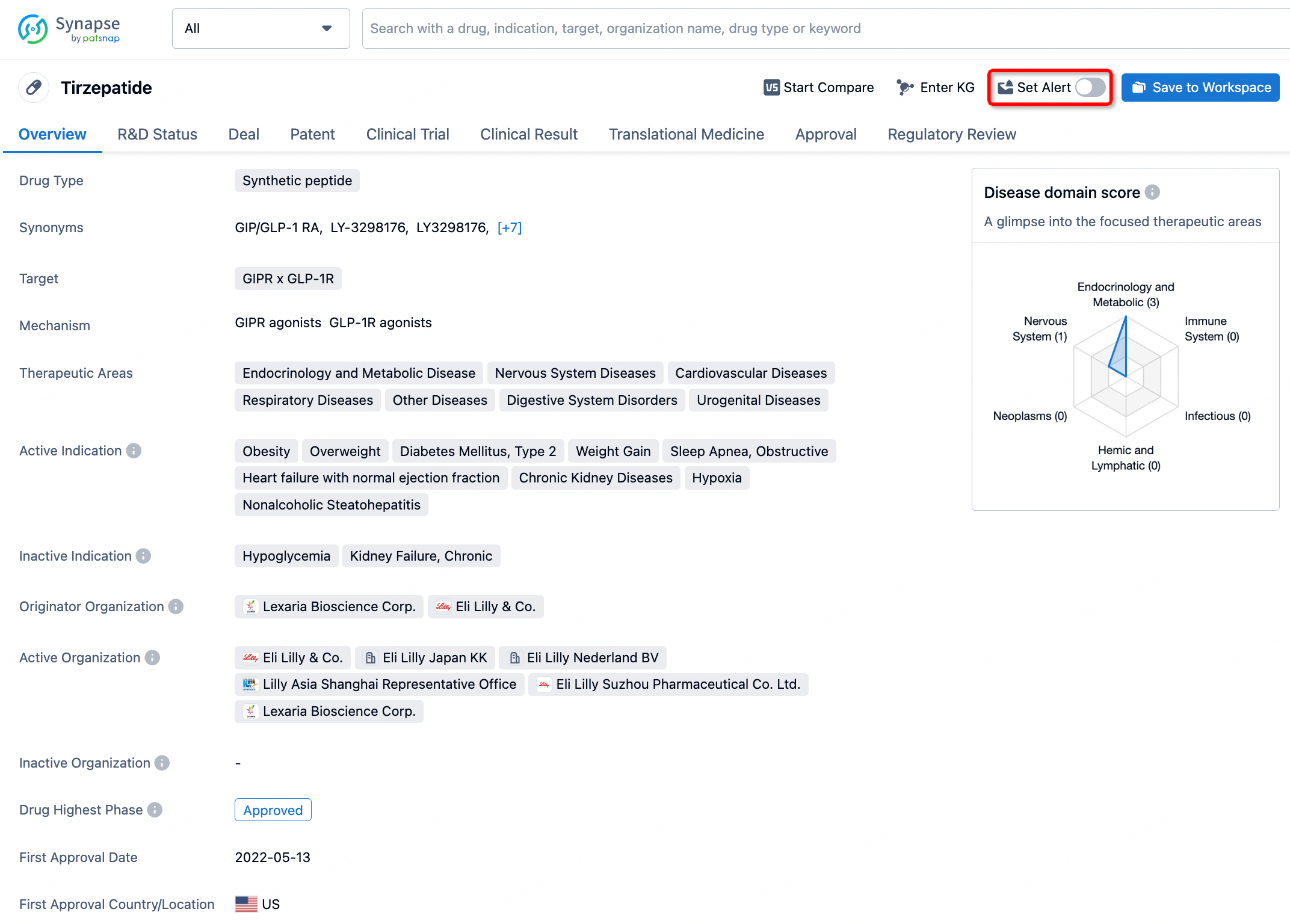
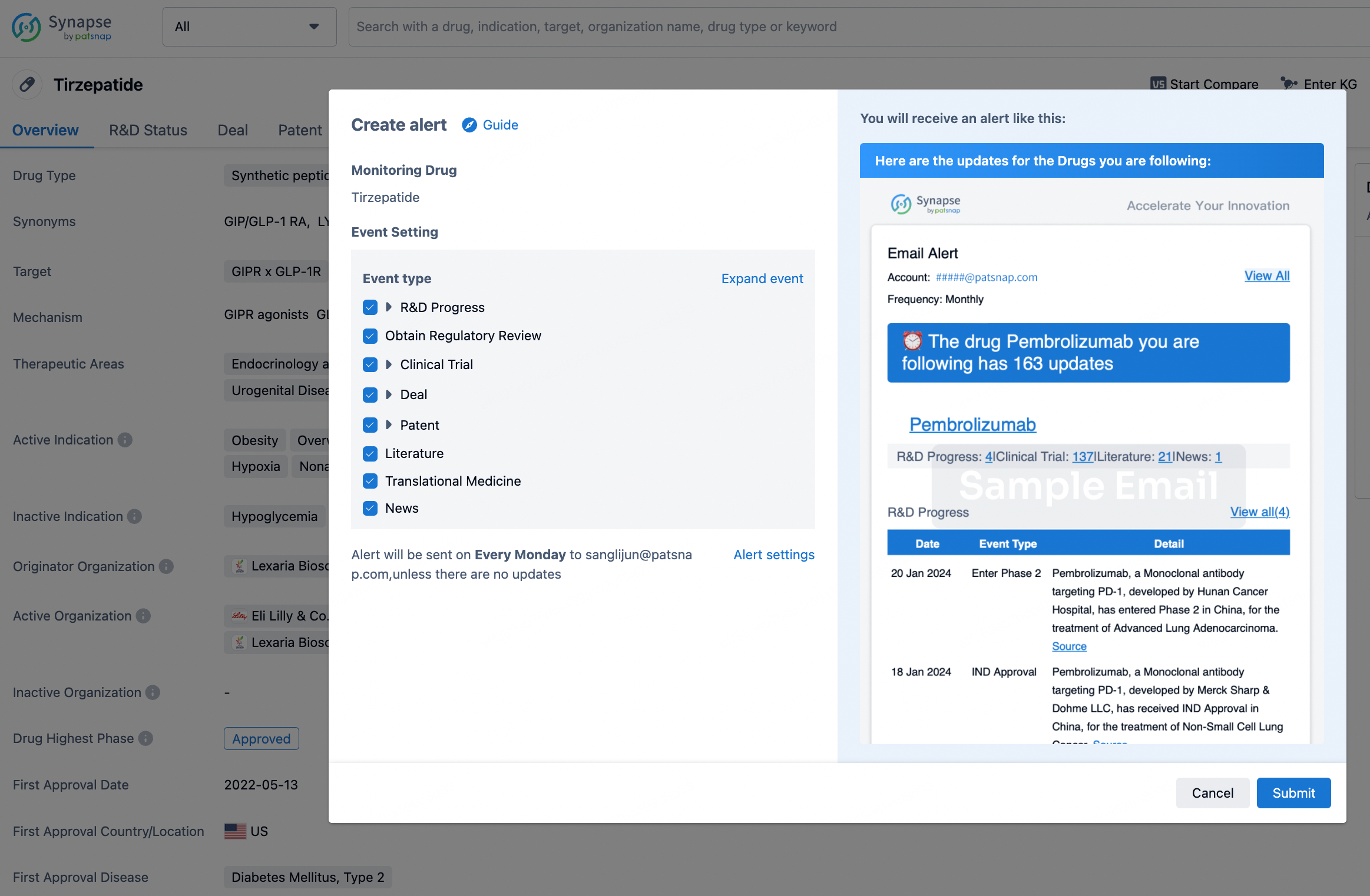
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!