Is Inmazeb approved by the FDA?
Yes, Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn) is an FDA-approved treatment for infections caused by the Zaire ebolavirus. Approved on October 14, 2020, it provides a crucial option for managing and treating Ebola virus disease.
What is Inmazeb?
Inmazeb is a combination of three monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn. This combination injection is used to treat infections caused by the Zaire ebolavirus.
How Inmazeb Works
Inmazeb works by targeting and neutralizing the Ebola virus. The three monoclonal antibodies in the combination bind to the glycoprotein on the surface of the Ebola virus, preventing the virus from entering and infecting human cells.
Administration and Dosage
- Dosage Form: Intravenous injection
- Administration: Administered by a healthcare professional through an IV over a period of 2 to 4 hours
- Frequency: As prescribed by a healthcare professional based on the patient's condition
Potential Side Effects
Common Side Effects:
- Injection site reactions (bleeding, blistering, burning, discoloration, itching, lumps, pain, rash, swelling, etc.)
- Diarrhea
Serious Side Effects:
- Infusion-related reactions (fever, chills, dizziness, headache, difficulty breathing, rash, lightheadedness, fainting, irregular heartbeat)
Patients should report any severe or unusual symptoms to their doctor immediately.
Warnings and Precautions
- Allergies: Inform your doctor of any allergies to medications, foods, dyes, preservatives, or animals.
- Vaccinations: Do not receive live vaccines during treatment and avoid close contact with individuals who have recently received live vaccines, as Inmazeb may lower your body's resistance to infections.
- Pregnancy and Breastfeeding: There are no adequate studies on the effects of Inmazeb during pregnancy or breastfeeding. Weigh the potential benefits against the potential risks.
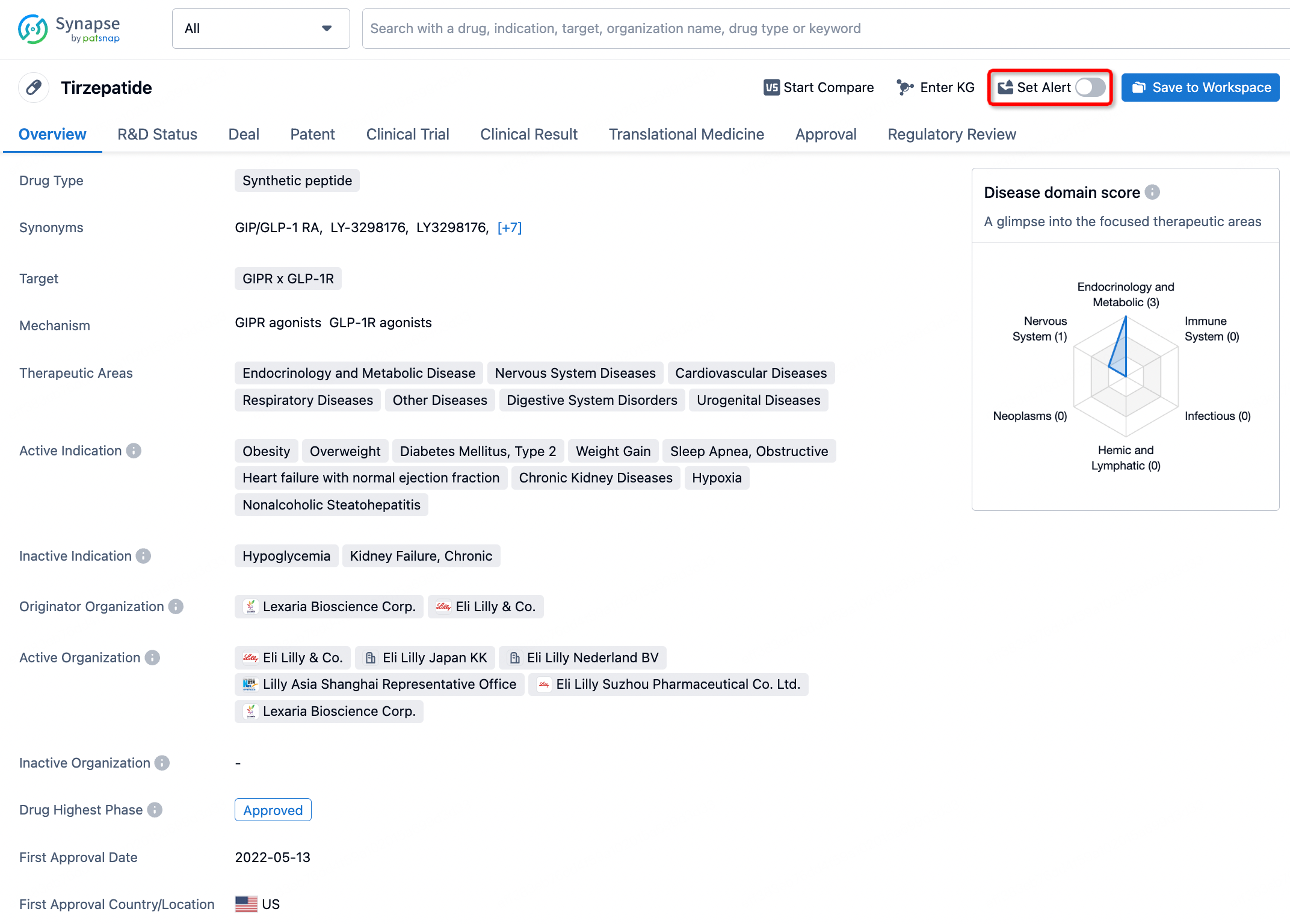
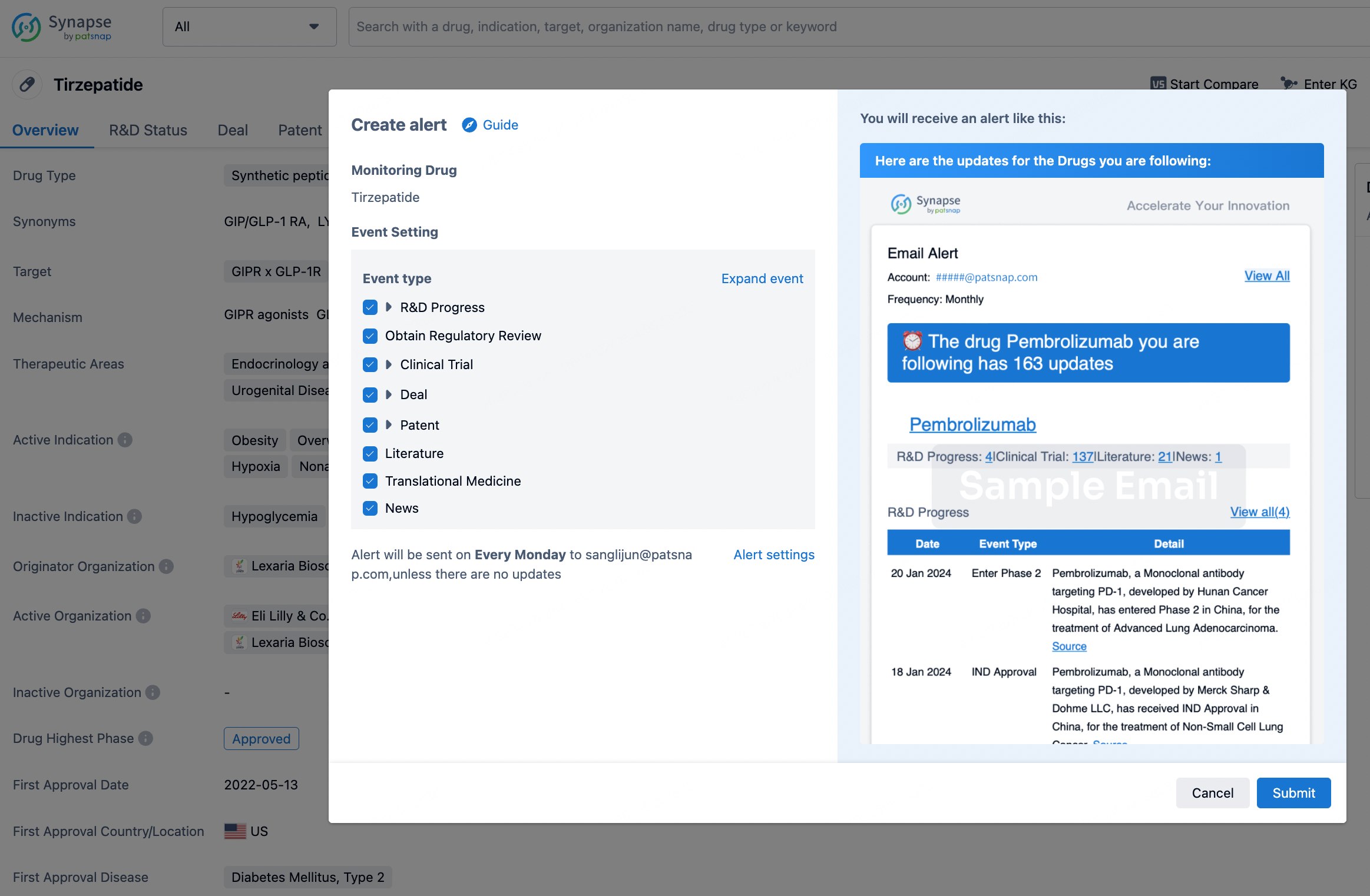
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!