Is Pralsetinib approved by the FDA?
Yes, pralsetinib (Gavreto) is an FDA-approved medication for treating RET fusion-positive non-small cell lung cancer and certain thyroid cancers. Approved on September 4, 2020, it represents a significant advancement in targeted cancer therapy.
What is Pralsetinib?
Pralsetinib is a multikinase inhibitor used to treat specific types of cancer, primarily non-small cell lung cancer (NSCLC) and certain types of thyroid cancer. It is specifically used for cancers that exhibit a genetic marker known as "RET" gene fusion. Your doctor will test for this genetic marker to determine if pralsetinib is appropriate for your treatment.
How Pralsetinib Works
Pralsetinib targets and inhibits the activity of certain proteins involved in the growth and spread of cancer cells, specifically those with RET gene fusions. By inhibiting these proteins, pralsetinib can help slow down or stop the progression of the cancer.
Administration and Dosage
- Dosage Form: Oral capsule (100 mg)
- Recommended Dose: 400 mg orally once daily on an empty stomach (at least 1 hour before or 2 hours after a meal)
- Duration: Continue until disease progression or unacceptable toxicity occurs
Potential Side Effects
Common Side Effects:
- High blood pressure
- Low blood cell counts or other abnormal laboratory tests
- Muscle or joint pain
- Fatigue
- Constipation
Serious Side Effects:
- Fever, chills
- New or worsening cough, shortness of breath, chest pain
- Severe headache, dizziness, confusion, trouble speaking
- Wound that will not heal
- Unusual bleeding or bruising
- Signs of bleeding inside the body (e.g., bloody or tarry stools, coughing up blood)
- Liver problems (e.g., nausea, vomiting, jaundice)
Patients should report any severe or unusual symptoms to their doctor immediately.
Warnings and Precautions
- Pregnancy and Birth Control: Pralsetinib can harm an unborn baby. Women should use effective non-hormonal birth control during treatment and for at least 2 weeks after the last dose. Men should use effective birth control if their partner can become pregnant, continuing for at least 1 week after the last dose.
- Breastfeeding: Do not breastfeed during treatment and for at least 1 week after the last dose.
- Pre-existing Conditions: Inform your doctor if you have a history of lung or breathing problems, bleeding issues, or high blood pressure.
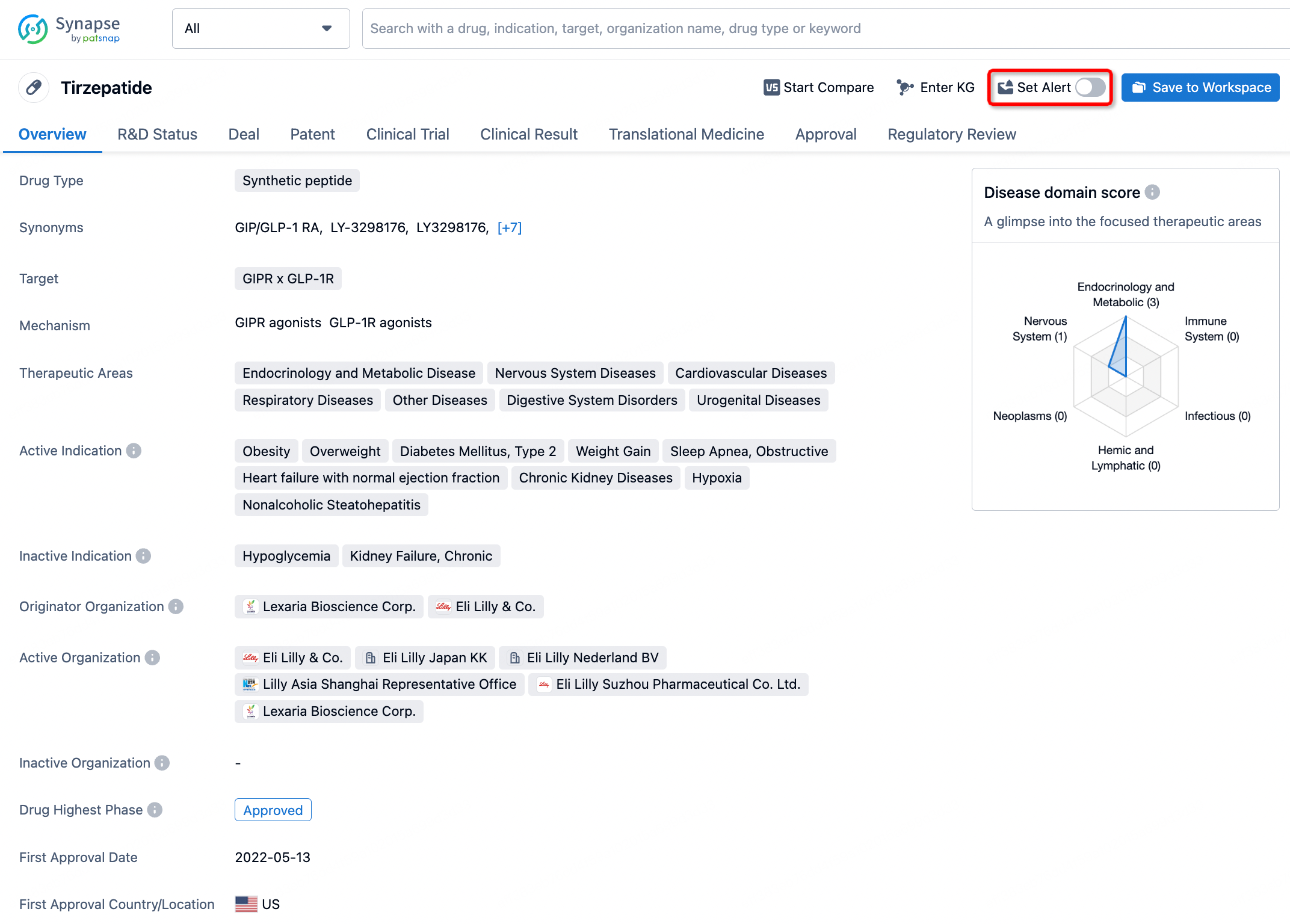
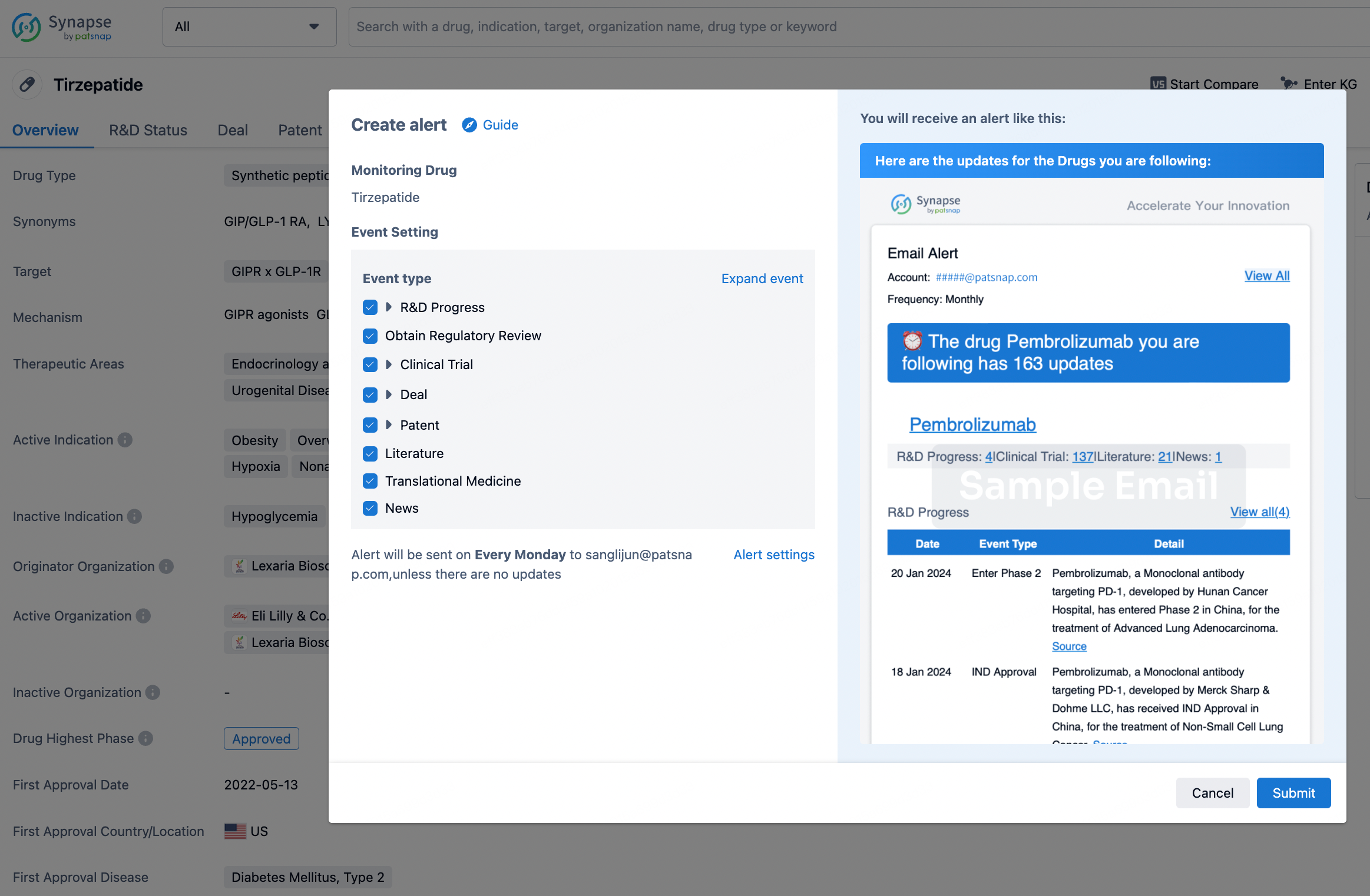
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!