Is Tezepelumab approved by the FDA?
Yes, tezepelumab (Tezspire) is FDA approved. The FDA granted approval for Tezspire on December 17, 2021, as an add-on maintenance treatment for severe asthma. This medication is specifically for patients whose asthma is not adequately controlled with their current medication regimen.
Uses
Tezepelumab is designed to be used in conjunction with other asthma medications to help control severe asthma. It is administered as a subcutaneous injection and is not intended for immediate relief of acute asthma attacks or bronchospasms. Patients should seek immediate medical attention if they experience worsening breathing problems or if their medications do not seem to be effective.
Dosage and Administration
- Adult and Pediatric Dosage: The recommended dose is 210 mg administered subcutaneously once every 4 weeks. This dosage applies to both adults and children aged 12 years and older.
- Administration: Tezepelumab is injected under the skin by a healthcare provider, and patients are monitored for a short time after each injection to ensure there is no allergic reaction.
- Missed Dose: If a dose is missed, patients should contact their doctor for instructions.
Side Effects
Common side effects of tezepelumab include:
- Sore throat
- Joint or back pain
Serious side effects that require immediate medical attention include signs of an allergic reaction, such as:
- Hives
- Difficulty breathing
- Swelling of the face, lips, tongue, or throat
- Dizziness, nausea, light-headedness, itching, or shortness of breath
Patients experiencing any unusual symptoms should contact their doctor for medical advice. Side effects can be reported to the FDA at 1-800-FDA-1088.
Warnings and Precautions
- Allergies: Patients should not use tezepelumab if they are allergic to it.
- Infections: Patients should inform their doctor if they have had a severe allergic reaction or a parasitic (helminth) infection, or if they have recently received or are scheduled to receive a live vaccine.
- Pregnancy and Breastfeeding: Patients should discuss with their doctor if they are pregnant, planning to become pregnant, or breastfeeding.
- Steroid Medication: Patients using steroid medications should not stop them suddenly and should follow their doctor's instructions for tapering the dose.
Drug Interactions
Tezepelumab may interact with other medications, including prescription and over-the-counter drugs, vitamins, and herbal products. Patients should inform their doctor about all medications they are taking.
Conclusion
Tezepelumab (Tezspire) is an FDA-approved medication for the maintenance treatment of severe asthma in patients 12 years and older. Approved on December 17, 2021, it is administered as a subcutaneous injection every 4 weeks. While effective in controlling severe asthma, patients should be aware of potential side effects and interactions with other medications. Always consult a healthcare provider for personalized medical advice and treatment plans.
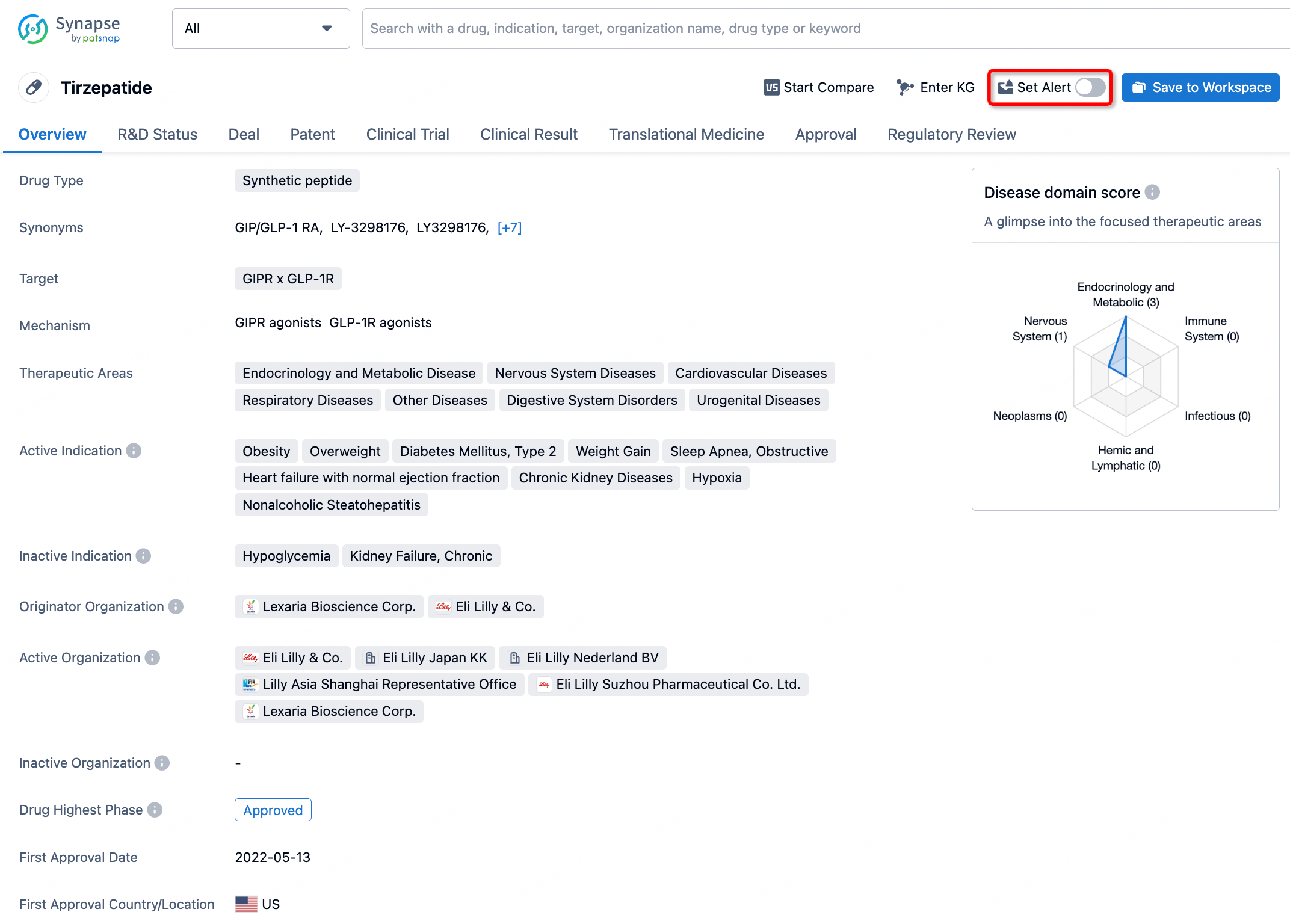
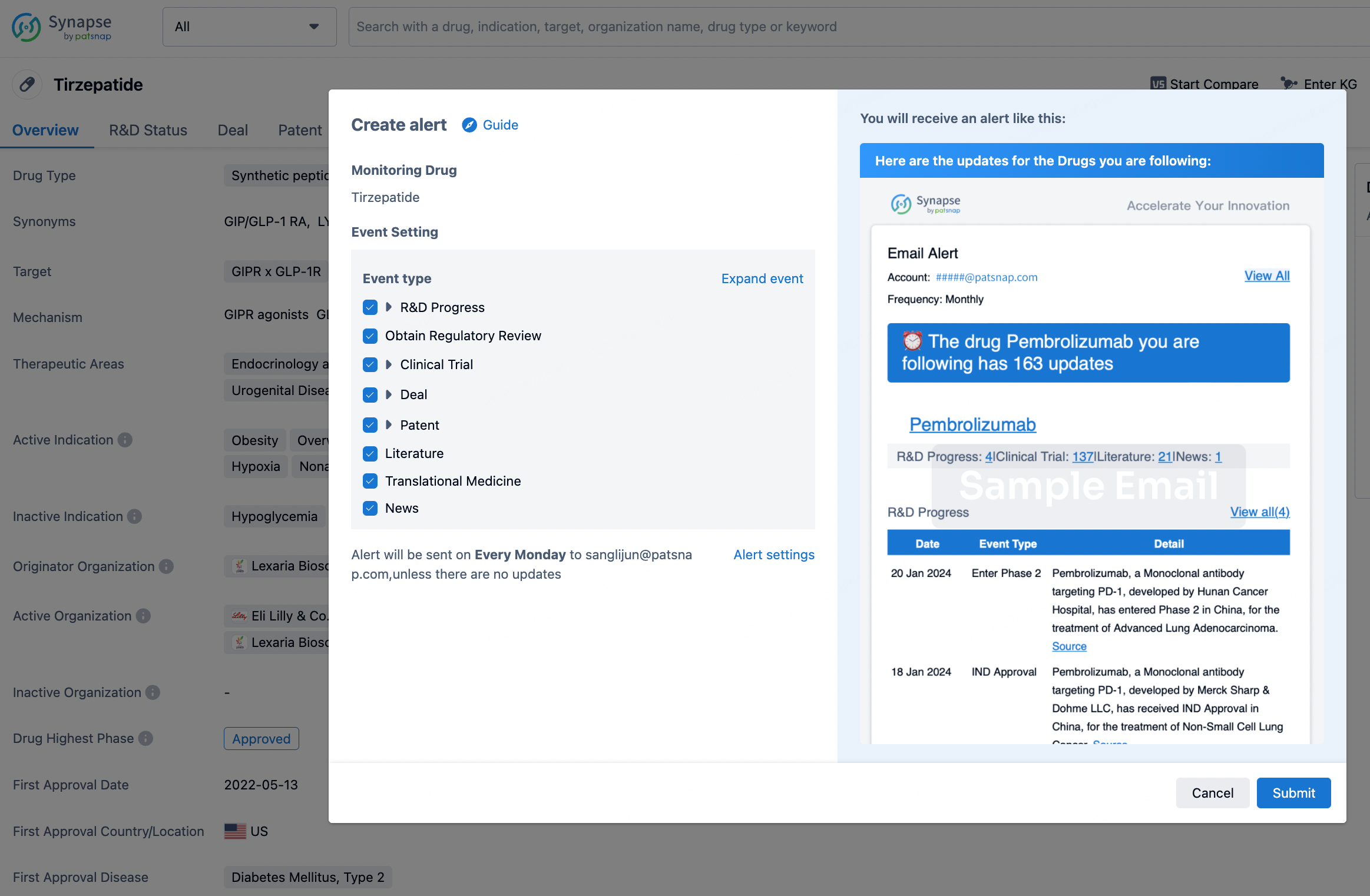
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!