Is Amivantamab approved by the FDA?
Amivantamab, marketed under the brand name Rybrevant, is a medication used to treat non-small cell lung cancer (NSCLC) that has an abnormal epidermal growth factor receptor (EGFR) gene. The U.S. Food and Drug Administration (FDA) approved amivantamab (Rybrevant) on May 21, 2021. This approval provided a new therapeutic option for patients with a specific type of NSCLC, marking a significant advancement in lung cancer treatment.
Mechanism of Action
Amivantamab is an intravenous solution that works by binding to the EGFR and MET receptors, which are involved in the growth and survival of cancer cells. By inhibiting these receptors, amivantamab can help to stop the growth and spread of cancer cells in patients with the specified EGFR mutation.
Administration and Dosage
Dosage Form: Amivantamab is administered as an intravenous solution (50 mg/mL).
Dosage Instructions:
- Initial Doses (Weeks 1 to 4):
- Patients weighing less than 80 kg: 1050 mg IV once a week.
- Patients weighing 80 kg or more: 1400 mg IV once a week.
- Subsequent Doses (Week 5 onwards):
- Patients weighing less than 80 kg: 1050 mg IV every 2 weeks.
- Patients weighing 80 kg or more: 1400 mg IV every 2 weeks.
The infusion is administered slowly over several hours, and premedication with antihistamines, antipyretics, and glucocorticoids is recommended to reduce the risk of infusion-related reactions.
Side Effects and Warnings
Common Side Effects:
- Reactions during the injection
- Cough, shortness of breath
- Fatigue
- Rash
- Infection around the nails
- Swelling
- Nausea, vomiting, constipation
- Mouth sores
- Joint or muscle pain
- Abnormal blood tests
Serious Side Effects:
- Eye pain or redness, sensitivity to light
- Vision changes, floaters
- Severe skin reactions
- Lung problems (new or worsening cough, fever, shortness of breath)
Warnings:
- Amivantamab can cause severe skin reactions; patients should avoid sunlight and tanning beds during treatment and for 2 months after the last dose. Protective clothing and sunscreen (SPF 30 or higher) are recommended.
- Patients may need frequent vision exams due to potential eye-related side effects.
Precautions
Before starting amivantamab, inform your healthcare provider if you have:
- Lung problems other than cancer
- Pregnancy or plan to become pregnant
- Breastfeeding
Women should use effective birth control during treatment and for at least 3 months after the last dose to avoid potential harm to the unborn baby. Breastfeeding should be avoided during treatment and for at least 3 months after the last dose.
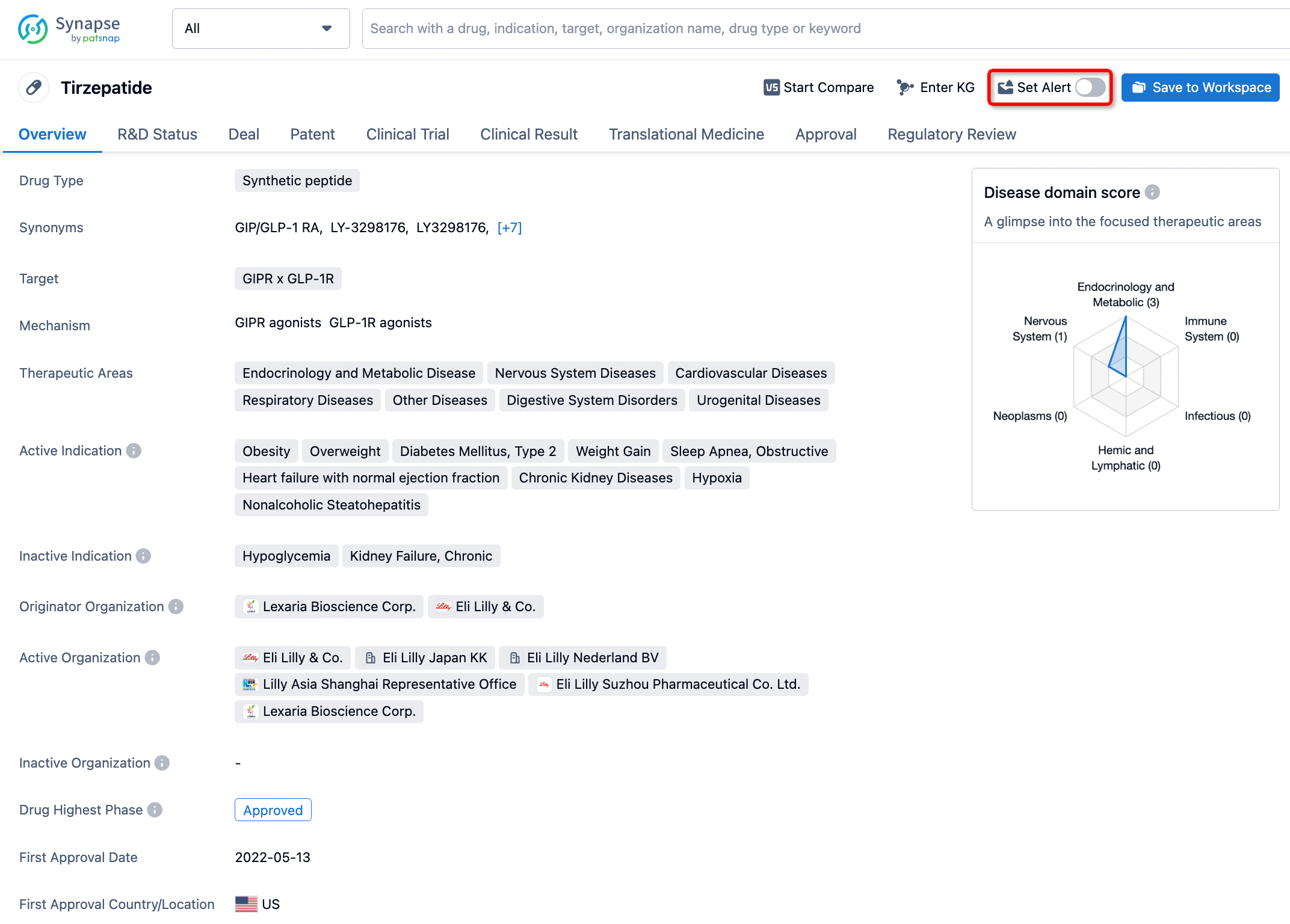
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!