Is Nivolumab/Relatlimab approved by the FDA?
Nivolumab and relatlimab received FDA approval for the treatment of unresectable or metastatic melanoma on March 18, 2022. This approval allows its use in patients meeting the specified criteria, aiming to manage advanced stages of melanoma where surgical removal isn't feasible.
What is Nivolumab and Relatlimab?
Nivolumab and relatlimab work together as immune checkpoint inhibitors, specifically targeting programmed cell death protein 1 (PD-1) and lymphocyte-activation gene 3 (LAG-3), respectively. By blocking these checkpoints, the drug combination enhances the body's immune response against cancer cells, thereby slowing their growth and spread.
Side Effects
Common side effects of nivolumab and relatlimab include muscle and bone pain, rash, itching, diarrhea, tiredness, and abnormal blood test results. However, it may also lead to serious adverse reactions such as immune-related complications affecting various organs, which require immediate medical attention.
Administration and Dosage
Nivolumab and relatlimab are administered intravenously every 4 weeks. The typical adult dose comprises 480 mg of nivolumab and 160 mg of relatlimab, tailored based on the patient's weight and tolerance. Treatment continues until disease progression or unacceptable toxicity develops, with regular monitoring through blood tests.
Precautions and Warnings
Before starting treatment, patients should inform their healthcare providers about any history of nervous system disorders, immune system problems, or recent transplants. Nivolumab and relatlimab can harm an unborn baby, necessitating effective contraception during treatment and for a specified period thereafter.
Conclusion
In conclusion, nivolumab and relatlimab, under the brand Opdualag, represent a significant advancement in the treatment landscape for unresectable or metastatic melanoma. Its FDA approval underscores its efficacy in managing this challenging condition, offering new hope to eligible patients.
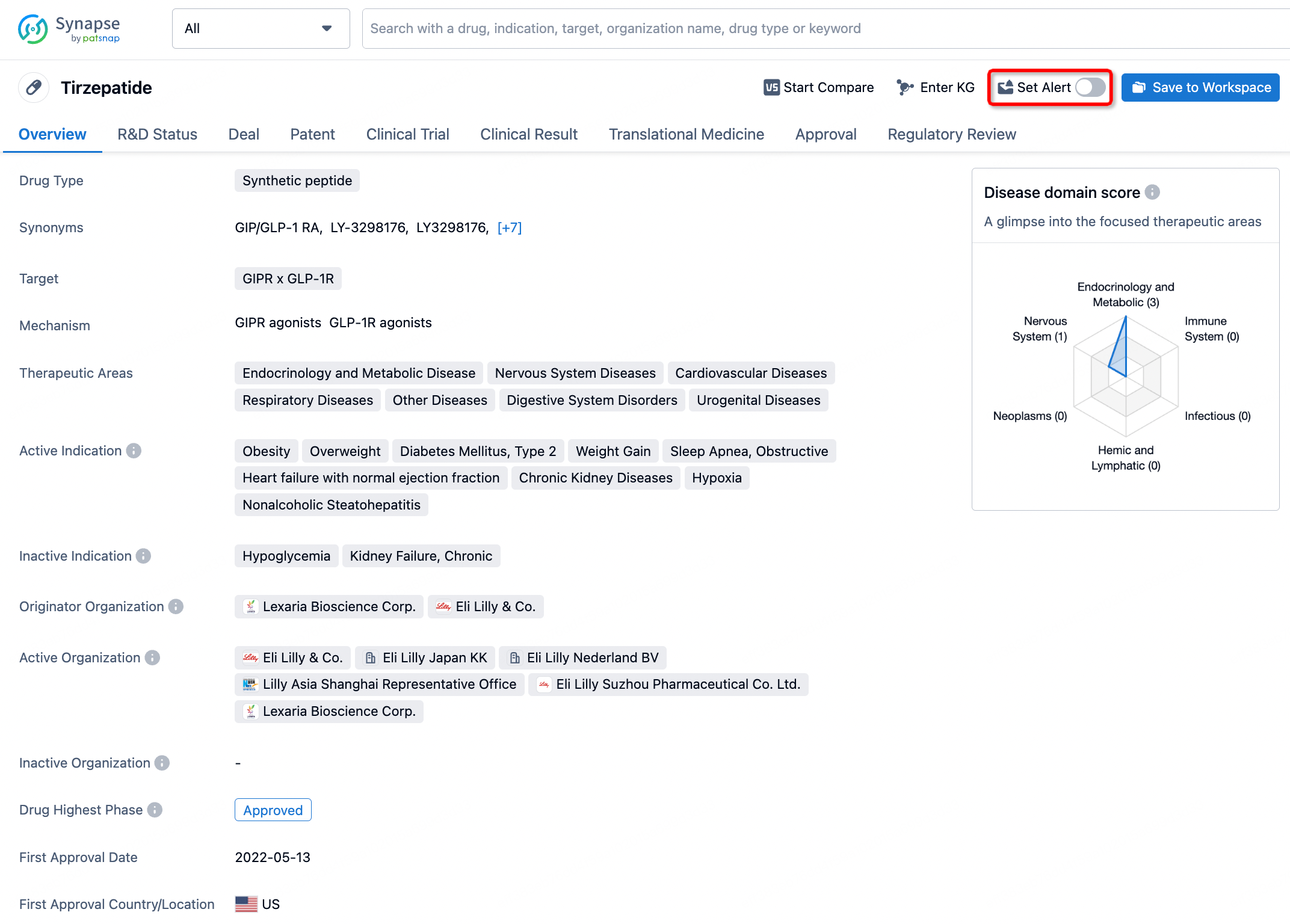
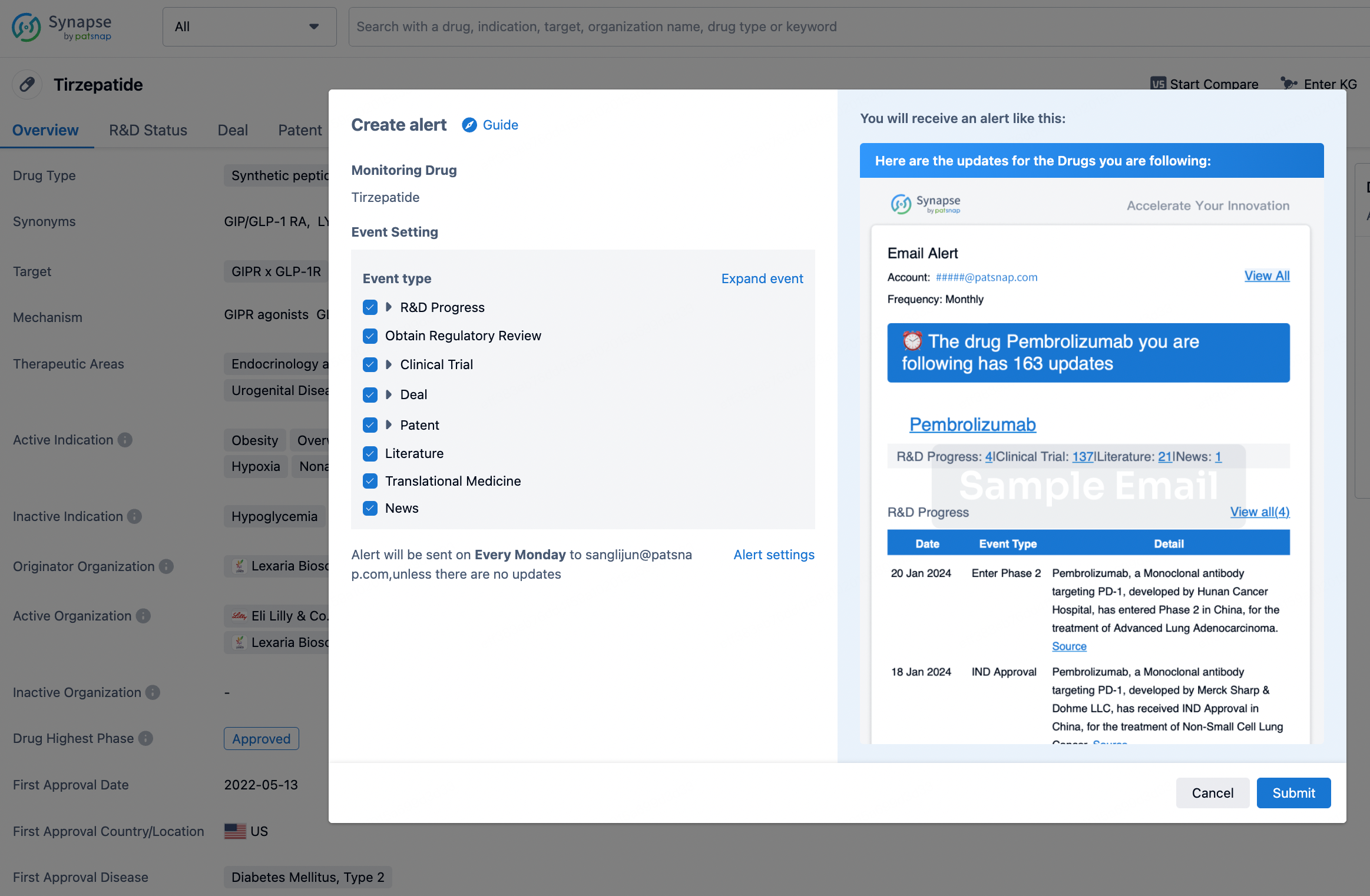
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!