Is Pegcetacoplan approved by the FDA?
Pegcetacoplan, marketed under the brand name Empaveli, is a selective immunosuppressant used to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).Pegcetacoplan (Empaveli) was approved by the U.S. Food and Drug Administration (FDA) on May 14, 2021, for the treatment of PNH. This approval marked a significant advancement in the management of this rare condition, providing a new therapeutic option for patients.
Mechanism of Action
Pegcetacoplan works by binding to the complement protein C3, which is part of the immune system. By preventing the cleavage of C3 into C3a and C3b, pegcetacoplan inhibits both intravascular hemolysis (IVH) and extravascular hemolysis (EVH). This dual-action mechanism helps to protect red blood cells from destruction in the bloodstream and in organs like the spleen, liver, and bone marrow, making it unique among PNH treatments.
Administration and Dosage
Dosage Form: Pegcetacoplan is administered via subcutaneous injection, using an infusion pump or the Empaveli injector.
Dosage Instructions:
- The recommended dosage is 1080 mg (one injection vial) administered subcutaneously twice weekly.
- Pegcetacoplan can be administered using an infusion pump, which usually takes about 30 minutes (using two sites) or 60 minutes (using one site).
- Alternatively, the Empaveli injector can be used for a more mobile infusion process.
Side Effects and Warnings
Common Side Effects:
- Injection-site reactions (redness, pain, swelling)
- Infections
- Diarrhea
- Abdominal pain
- Limb pain
- Low potassium levels
- Fatigue
- Cough
- Joint pain
- Dizziness
- Headache
- Rash
Serious Side Effects:
- Serious infections by encapsulated bacteria
- Allergic reactions during infusion, such as chest pain, trouble breathing, and swelling of the face, tongue, or throat
Warnings:
- Pegcetacoplan can increase the risk of serious and life-threatening infections, including meningitis caused by bacteria such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. Vaccination against these bacteria is required at least two weeks before starting treatment.
- Pegcetacoplan may cause infusion-related reactions or anaphylaxis, which can be life-threatening.
- Patients should carry an Empaveli Patient Safety Card to alert healthcare providers about the risk of serious infections.
Precautions
Before starting pegcetacoplan, inform your healthcare provider if you:
- Have an infection or fever
- Have not received all childhood vaccinations
- Are pregnant, planning to become pregnant, or breastfeeding
Conclusion
Its unique mechanism of action and targeted approach to preventing red blood cell destruction offer hope for better disease management. As with any medication, it is essential to be aware of potential side effects and to follow your healthcare provider's guidance closely.
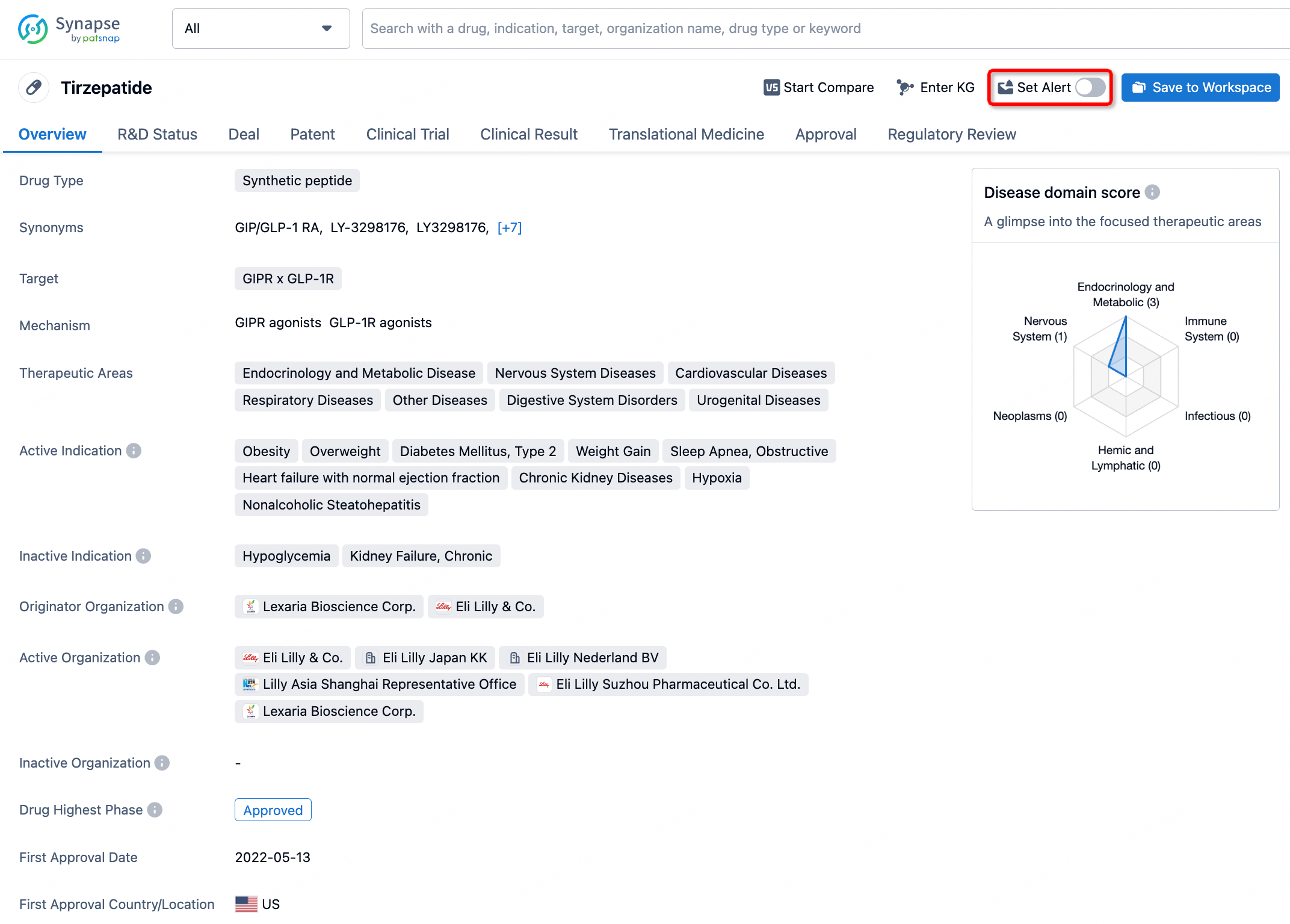
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!