iTeos Announces First Patient Dosed in Phase 3 GALAXIES Lung-301 Trial, Receives $35 Million from GSK
iTeos Therapeutics, Inc., a biopharmaceutical company in the clinical stage known for its innovative work in immuno-oncology therapeutics, has announced that the first patient has been dosed in the GALAXIES Lung-301 trial. This is an international, randomized, double-blind Phase 3 clinical trial aimed at registration, comparing belrestotug + dostarlimab against placebo + pembrolizumab in patients with advanced, unresectable, or metastatic PD-L1 high NSCLC as their first-line treatment. This milestone activates $35 million in development milestone payments from their partner GSK for belrestotug.
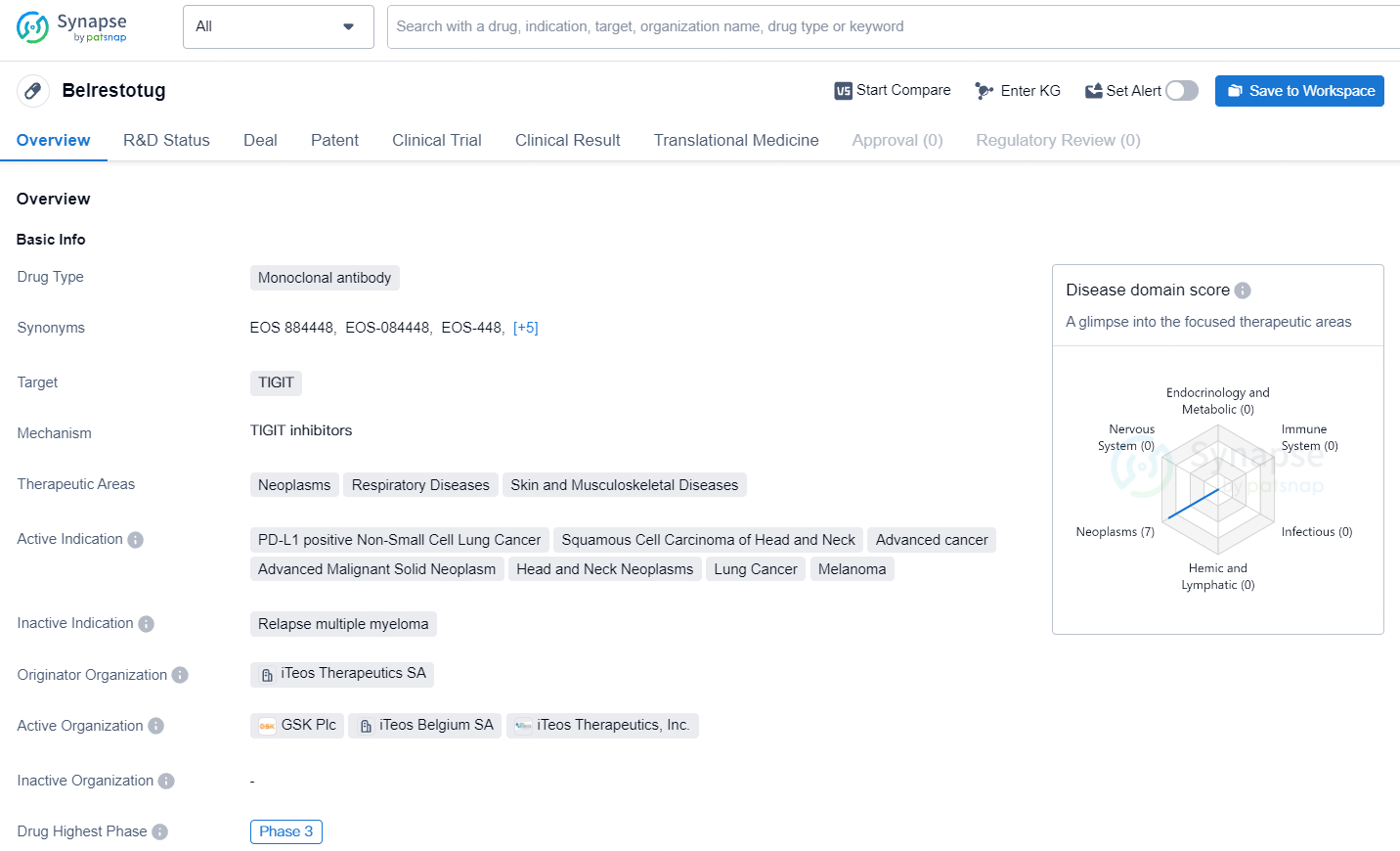
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Administering the first dose to a patient in the GALAXIES Lung-301 study represents a critical achievement for belrestotug + dostarlimab. iTeos was established with the objective of enhancing therapeutic choices for patients through top-tier scientific research. Initiating our inaugural TIGIT: PD-1 doublet Phase 3 trial highlights our stringent, data-driven investment methodology and was guided by encouraging preliminary clinical indicators such as safety, ORR, and response depth from the latest interim analysis in May 2024,” commented Michel Detheux, Ph.D., president and CEO of iTeos.
"We are enthusiastic about the continued Phase 2 GALAXIES Lung-201 trial and look forward to sharing findings from the recent interim analysis of this study at a 2024 medical conference," Michel Detheux continued.
In June 2021, iTeos and GSK formed an exclusive partnership for the development and commercialization of belrestotug, an anti-TIGIT monoclonal antibody, enabling advanced next-generation immuno-oncology combinations. According to the agreement, iTeos received an initial $625 million payment from GSK, with potential development and regulatory milestones up to $550 million and commercial milestones up to $900 million. Additionally, GSK and iTeos will jointly commercialize and equally share profits in the US. Outside the US, GSK will have exclusive commercialization rights and iTeos will receive tiered royalty payments. The global development of belrestotug will be a shared responsibility and cost for both GSK and iTeos.
Belrestotug is an Fc-active human immunoglobulin G1 (IgG1) monoclonal antibody targeting TIGIT, a significant inhibitory receptor involved in the downregulation of innate immune responses to cancer. Designed to be an optimized, high-affinity, potent anti-TIGIT mAb, belrestotug aims to enhance antitumor activity through a multifaceted immune modulation mechanism by interacting with TIGIT and FcγR. This key regulator of immune responses induces cytokine release and antibody-dependent cellular cytotoxicity. The therapeutic candidate is being advanced in multiple indications in collaboration with GSK.
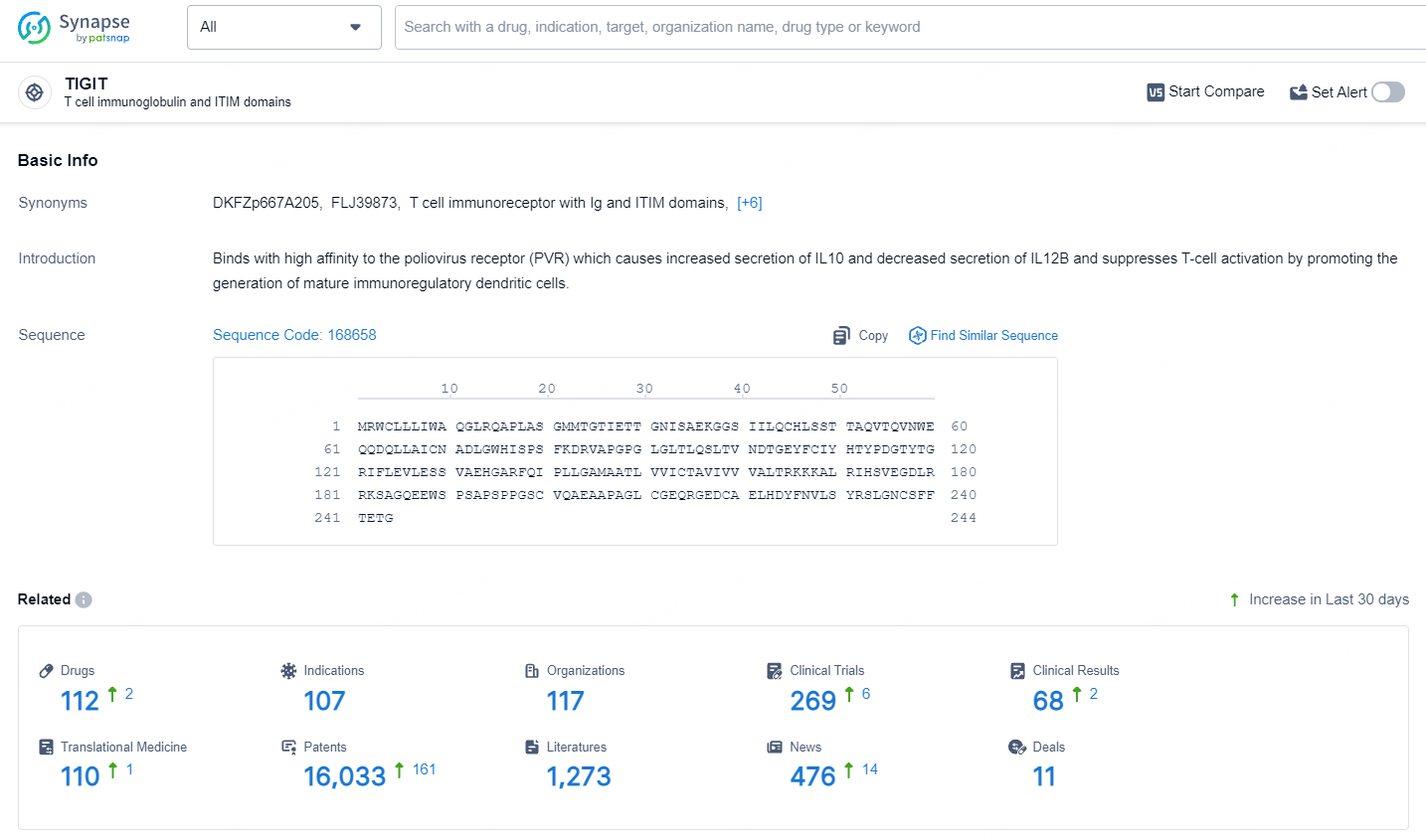
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 10, 2024, there are 112 investigational drugs for the TIGIT target, including 107 indications, 117 R&D institutions involved, with related clinical trials reaching 269, and as many as 16033 patents.
Belrestotug represents an important advancement in the field of biomedicine, particularly in the area of immunotherapy for cancer. As the drug progresses through Phase 3 clinical trials, further data will be generated to evaluate its safety and efficacy, with the potential to address unmet medical needs in the treatment of neoplasms, respiratory diseases, and skin and musculoskeletal diseases.