Ixekizumab Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs, Mechanisms of Action, and Drug Target
Ixekizumab's R&D Progress
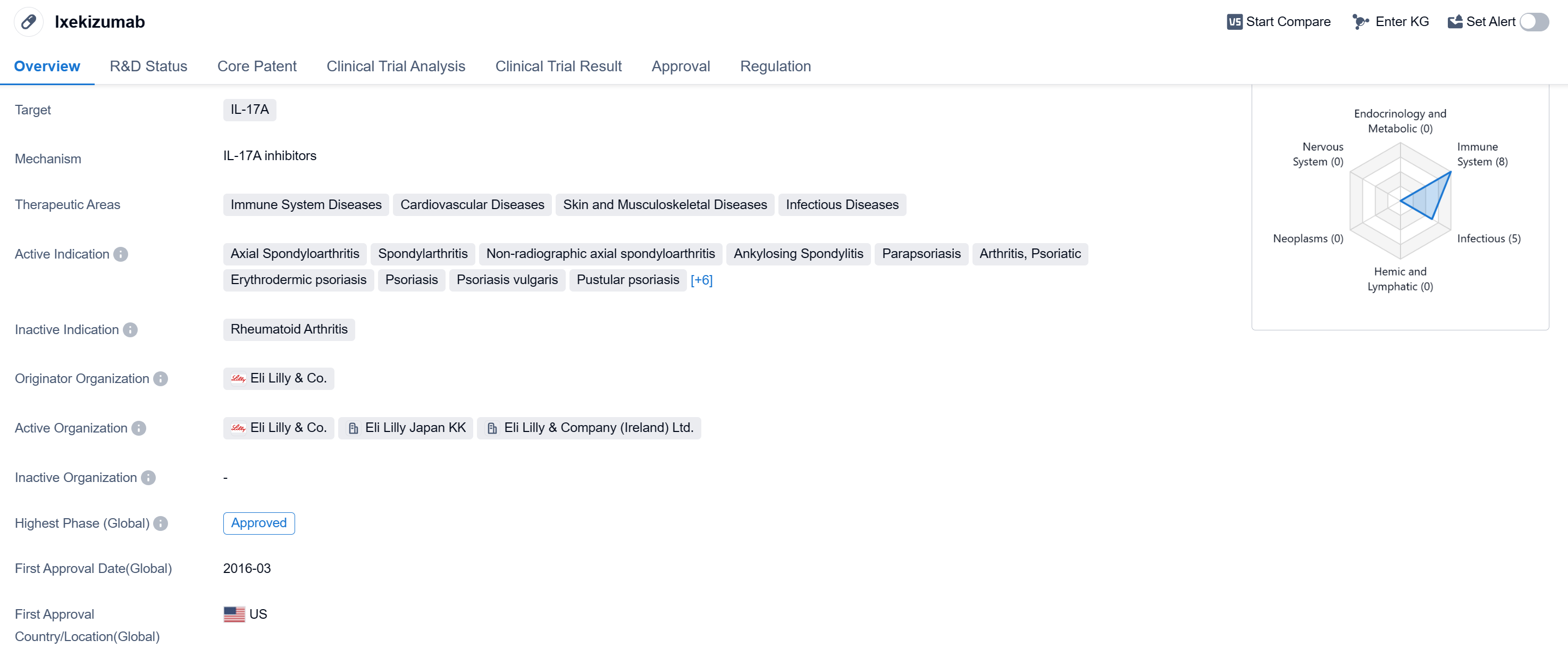
Ixekizumab is a monoclonal antibody drug that targets IL-17A, making it a promising treatment option for various immune system diseases, cardiovascular diseases, skin and musculoskeletal diseases, as well as infectious diseases. The drug has been approved for multiple indications globally and in China.
In terms of therapeutic areas, Ixekizumab has shown efficacy in treating conditions such as axial spondyloarthritis, spondylarthritis, non-radiographic axial spondyloarthritis, ankylosing spondylitis, parapsoriasis, arthritis psoriatic, erythrodermic psoriasis, psoriasis, psoriasis vulgaris, pustular psoriasis, plaque psoriasis, juvenile arthritis, varicose ulcer, pityriasis rubra pilaris, pemphigoid bullous, and pyoderma gangrenosum.
The drug was developed by Eli Lilly & Co., a renowned pharmaceutical company. It received its first approval in the United States in March 2016, marking a significant milestone for the company. The approval was granted after a priority review, indicating the drug's potential to address unmet medical needs.
Ixekizumab's approval in China further highlights its global recognition and potential impact on patient care. The drug has been classified as an overseas new drug urgently needed in clinical settings, emphasizing its importance in the Chinese market.
As a monoclonal antibody, Ixekizumab offers a targeted approach to treating diseases by specifically inhibiting IL-17A. This mechanism of action has shown promising results in clinical trials, leading to its approval for various indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ixekizumab: IL-17A inhibitors
IL-17A inhibitors are a type of medication that target and block the activity of interleukin-17A (IL-17A), a cytokine involved in inflammation and immune responses. From a biomedical perspective, IL-17A inhibitors are used in the treatment of various autoimmune diseases, particularly those characterized by excessive inflammation, such as psoriasis, psoriatic arthritis, and ankylosing spondylitis.
IL-17A is produced by immune cells and plays a role in promoting inflammation and recruiting other immune cells to the site of inflammation. However, in certain autoimmune diseases, the production of IL-17A is dysregulated, leading to chronic inflammation and tissue damage. IL-17A inhibitors work by binding to IL-17A or its receptor, preventing its interaction with target cells and thereby reducing inflammation.
By inhibiting IL-17A, these medications help to alleviate symptoms and slow down the progression of autoimmune diseases. They can reduce skin inflammation, joint pain, and stiffness in conditions like psoriasis and psoriatic arthritis. IL-17A inhibitors are typically administered as injections or infusions and are prescribed under the guidance of a healthcare professional.
It's important to note that IL-17A inhibitors may have potential side effects, including increased risk of infections, so close monitoring and regular follow-up with a healthcare provider are necessary during treatment.
Drug Target R&D Trends for Ixekizumab
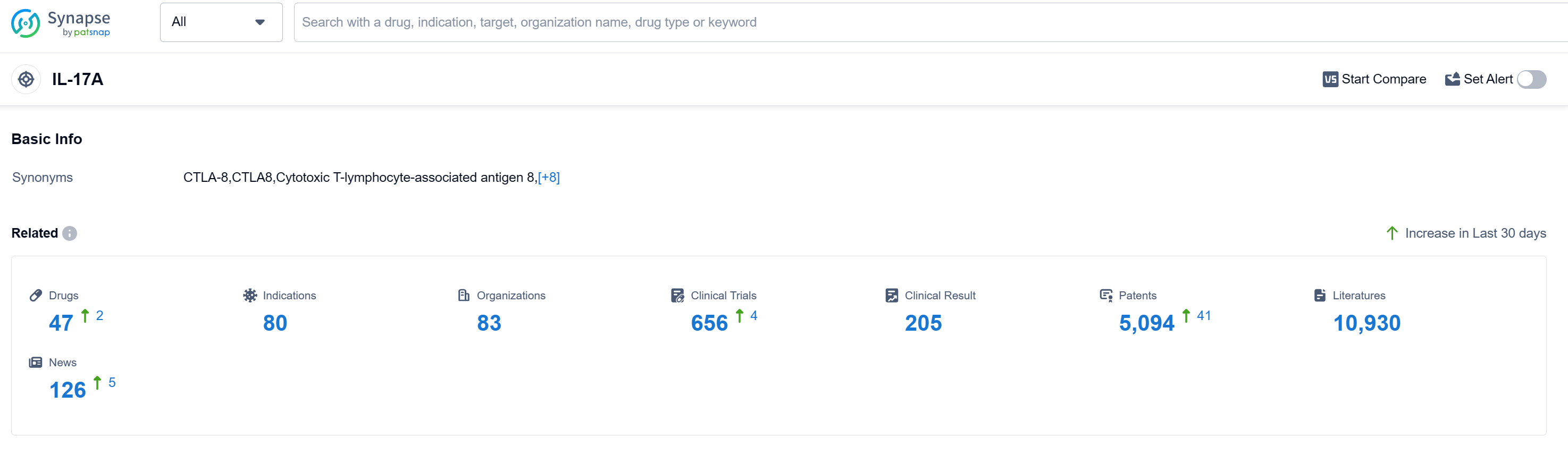
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 47 IL-17A drugs worldwide, from 83 organizations, covering 80 indications, and conducting 656 clinical trials.
The analysis of the current competitive landscape and future development of target IL-17A reveals that companies such as Novartis AG, UCB SA, and Eli Lilly & Co. are growing fastest under this target. These companies have drugs in various stages of development, including approved, NDA/BLA, and different phases of clinical trials.
Drugs targeting IL-17A have been approved for indications such as plaque psoriasis, arthritis (psoriatic, ankylosing spondylitis, axial spondyloarthritis), pustular psoriasis, and others. Monoclonal antibodies and biosimilars are the most rapidly progressing drug types under this target, indicating intense competition in the market.
Countries/locations such as China, the United States, Japan, and the European Union are developing rapidly under the target IL-17A, with drugs in approved and preclinical stages. China, in particular, has shown significant progress in the development of drugs targeting IL-17A.
Overall, the target IL-17A presents a competitive landscape with multiple companies, various indications, and different drug types. The future development of IL-17A will likely involve further research and development, clinical trials, and regulatory approvals to bring innovative therapies to patients.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Ixekizumab's approval and therapeutic potential in multiple disease areas make it a valuable addition to the pharmaceutical industry. Its success in the global markets reflects its efficacy and potential to improve patient outcomes. As the drug continues to be utilized in clinical settings, further research and development may uncover additional indications and expand its impact on patient care.