Unleashing the Power of Inclisiran: A Comprehensive Review on R&D Breakthroughs, Action Mechanisms, and Drug Target
Inclisiran's R&D Progress
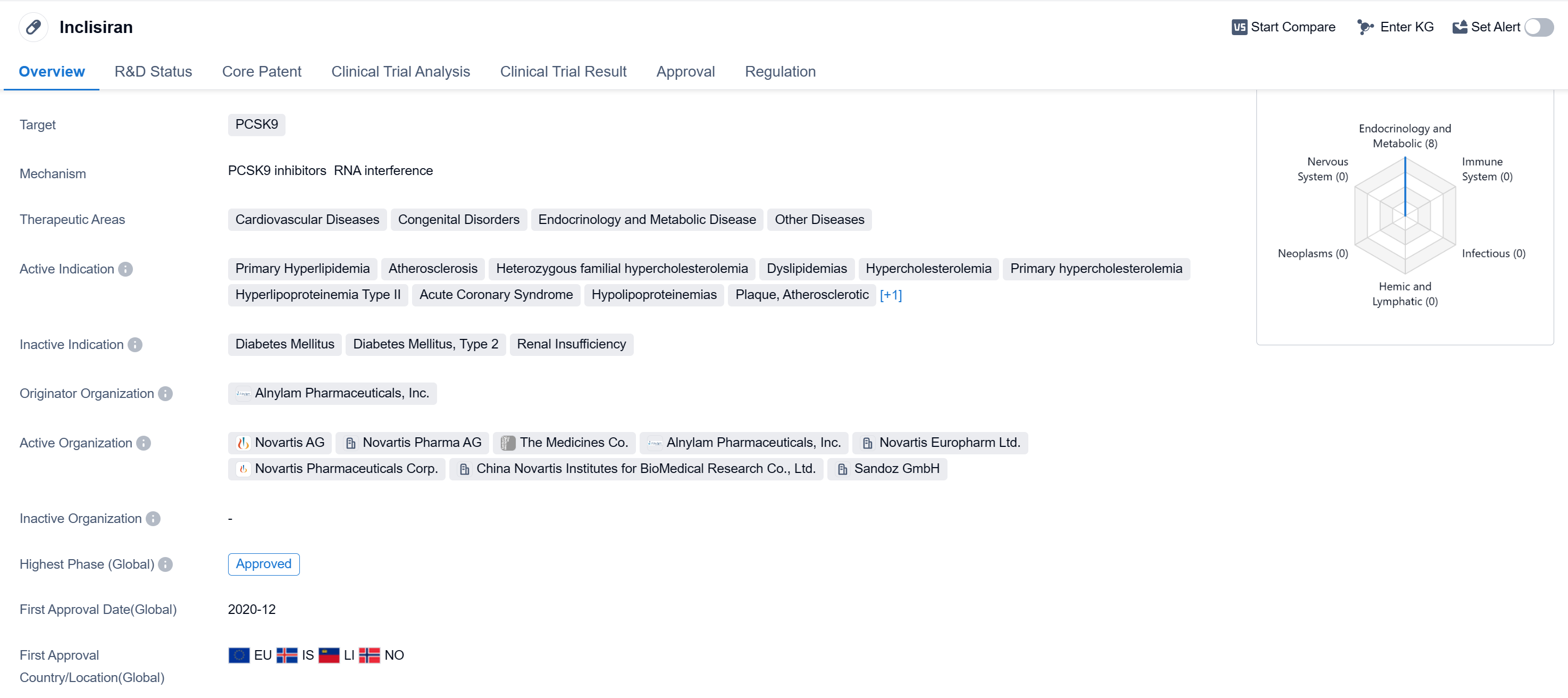
Inclisiran is a drug classified as a small interfering RNA and is primarily used to target PCSK9. It is indicated for the treatment of various cardiovascular diseases, congenital disorders, endocrinology and metabolic diseases, as well as other diseases. The active indications for Inclisiran include primary hyperlipidemia, atherosclerosis, heterozygous familial hypercholesterolemia, dyslipidemias, hypercholesterolemia, primary hypercholesterolemia, hyperlipoproteinemia Type II, acute coronary syndrome, hypolipoproteinemias, plaque, atherosclerotic, and homozygous familial hypercholesterolemia.
The drug was developed by Alnylam Pharmaceuticals, Inc., an originator organization in the pharmaceutical industry. In terms of its development phase, Inclisiran has reached the highest phase of approval globally. It received its first approval in December 2020 in the European Union, Iceland, Liechtenstein, and Norway.
In terms of regulatory status, Inclisiran is classified as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This classification provides certain incentives and benefits to the manufacturer, such as market exclusivity and financial incentives, to encourage the development of treatments for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Inclisiran: PCSK9 inhibitors RNA interference
PCSK9 inhibitors are a type of medication used in biomedicine to lower cholesterol levels in the body. PCSK9 stands for proprotein convertase subtilisin/kexin type 9, which is an enzyme involved in regulating the amount of low-density lipoprotein (LDL) cholesterol in the bloodstream. High levels of LDL cholesterol can increase the risk of cardiovascular diseases such as heart attacks and strokes.
PCSK9 inhibitors work by blocking the action of the PCSK9 enzyme, which helps to increase the number of receptors on liver cells that remove LDL cholesterol from the blood. By inhibiting PCSK9, these medications can effectively lower LDL cholesterol levels and reduce the risk of cardiovascular events.
RNA interference, on the other hand, is a biological process that occurs naturally in cells to regulate gene expression. It involves the use of small RNA molecules to silence or "interfere" with the expression of specific genes. This process plays a crucial role in various cellular functions and can be harnessed for therapeutic purposes.
In the context of biomedicine, RNA interference (RNAi) has been extensively studied as a potential tool for targeted gene therapy. By designing small interfering RNA (siRNA) molecules that are complementary to specific disease-causing genes, researchers can selectively silence those genes and potentially treat various genetic disorders, viral infections, and even certain types of cancer.
In summary, PCSK9 inhibitors are medications used to lower cholesterol levels by blocking the action of the PCSK9 enzyme, while RNA interference is a natural biological process that can be utilized for targeted gene therapy in biomedicine.
Drug Target R&D Trends for Inclisiran
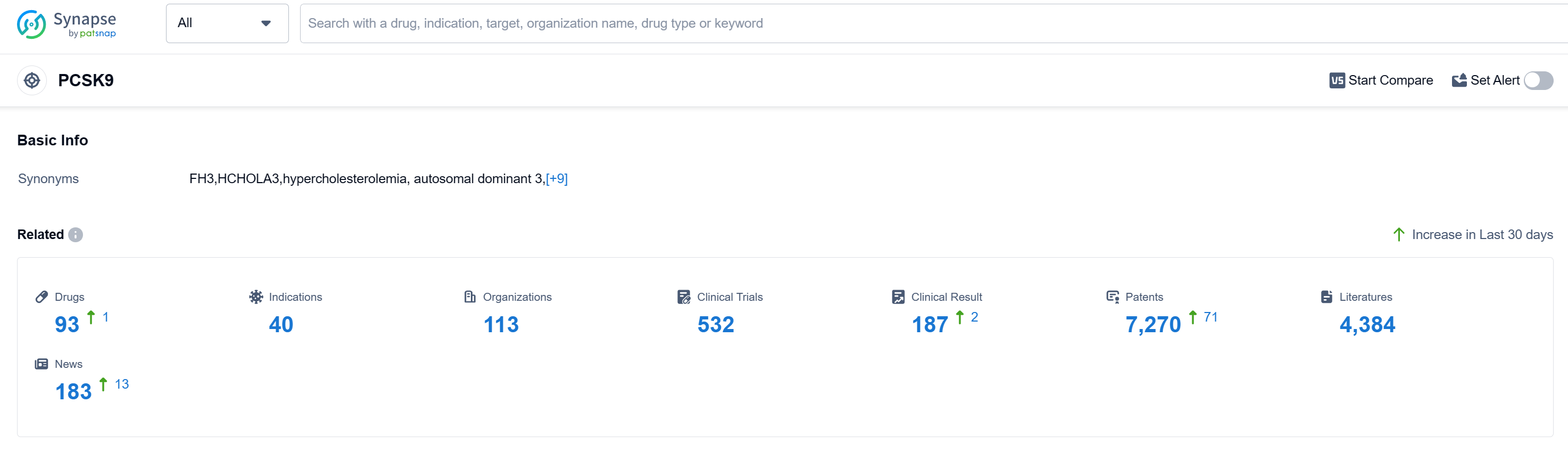
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 93 PCSK9 drugs worldwide, from 113 organizations, covering 40 indications, and conducting 532 clinical trials.
The analysis of the target PCSK9 reveals a competitive landscape with multiple companies actively involved in research and development. Novartis AG, Amgen, Inc., Regeneron Pharmaceuticals, Inc., and Sanofi are leading in terms of the highest stage of development. The drugs under the target PCSK9 have been approved for various indications, indicating their potential in treating dyslipidemias, hypercholesterolemia, atherosclerosis, and other related conditions.
Monoclonal antibodies are the most rapidly progressing drug type, suggesting intense competition in the market. Biosimilars are being developed by various research and development institutions, including Novartis AG, Amgen, Inc., Regeneron Pharmaceuticals, Inc., Sanofi, Innovent Biologics, Inc., and Takeda Pharmaceutical Co., Ltd.
China, the United States, and the European Union are the countries/locations with the fastest development under the target PCSK9. China, in particular, has made significant progress in the development of drugs for PCSK9-related conditions.
Overall, the target PCSK9 presents a competitive landscape with promising future development. The ongoing research and development efforts by various companies and countries indicate a growing interest in advancing the treatment options for PCSK9-related conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Inclisiran is a small interfering RNA drug developed by Alnylam Pharmaceuticals, Inc. It targets PCSK9 and is indicated for the treatment of various cardiovascular diseases, congenital disorders, endocrinology and metabolic diseases, and other diseases. It has received approval in multiple countries, including the European Union, Iceland, Liechtenstein, and Norway. The drug is classified as an orphan drug, indicating its focus on treating rare diseases or conditions.