J&J Seeks FDA Approval for DARZALEX FASPRO in Treating Newly Diagnosed Multiple Myeloma Cases
Pharmaceutical giant Johnson & Johnson has officially filed a supplemental request with the U.S. Food and Drug Administration (FDA) for an expanded use of their product, DARZALEX FASPRO (daratumumab and hyaluronidase-fihj). This application is specific to proposing DARZALEX FASPRO to be used in tandem with a regimen consisting of bortezomib, lenalidomide, and dexamethasone as part of an initial and follow-up therapy. Additionally, it is intended to be used alongside lenalidomide for the continued treatment of adult patients who have recently been diagnosed with multiple myeloma and who meet the criteria for undergoing autologous stem cell transplantation.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.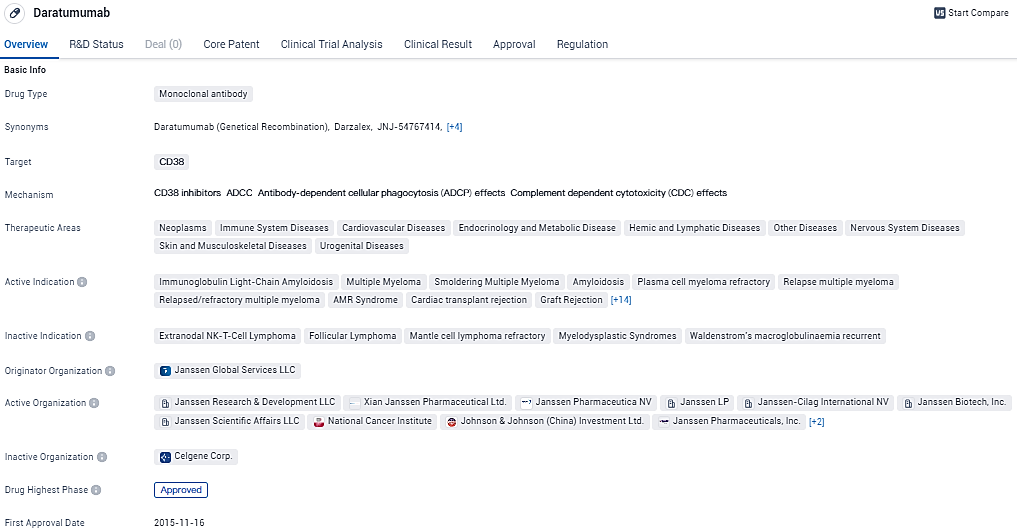
The data submission for this application draws on results from the Phase 3 PERSEUS trial which examined the efficacy of D-VRd as induction and consolidation therapy alongside ASCT, followed by a D-R maintenance regimen. This was measured against a regimen comprising bortezomib, lenalidomide, dexamethasone, ASCT, and lenalidomide-only maintenance. The key finding from the core analysis indicated that the trial reached its main goal of improving progression-free survival, with a notable 58 percent decrease in the likelihood of disease advancement or mortality.
The regimen involving D-VRd, succeeded by ASCT and continued with D-R for maintenance purposes, was found to enhance response quality, observed in heightened instances of complete or better responses. This included a more stringent complete response and a greater frequency of patients reaching a state of negligible minimal residual disease when compared to the VRd, ASCT, and R maintenance protocol.
In the cohort proceeding to the maintenance stage within the D-VRd group, 64 percent were able to cease treatment with DARZALEX FASPRO having attained a complete response or an enhanced response state, along with consistent minimal residual disease-negativity after a minimum two-year period on the D-R maintenance therapy, adhering to study directions. The adverse event profile for patients on D-VRd, followed by the D-R schedule, aligned with those previously established for DARZALEX FASPRO, VRd, and R.
Dr. Craig Tendler, Vice President of Clinical Development, Diagnostics, and Global Medical Affairs at Johnson & Johnson Innovative Medicine, expressed the company's dedication to altering the prognosis for patients with multiple myeloma via strategic combinations like D-VRd, that leverage synergistic modes of action. The novel approach using DARZALEX FASPRO-based quadruplet therapy has been shown to significantly diminish the likelihood of disease progression or death in freshly diagnosed patients eligible for transplants.
Dr. Tendler further highlighted the importance of initiating treatment with D-VRd for patients, pointing out the regimen's capacity to yield robust and lasting therapeutic responses from the outset. He emphasized the possibility of enhancing long-term results for those recently diagnosed and expressed eagerness to engage with the FDA during the reviewal phase of this application.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
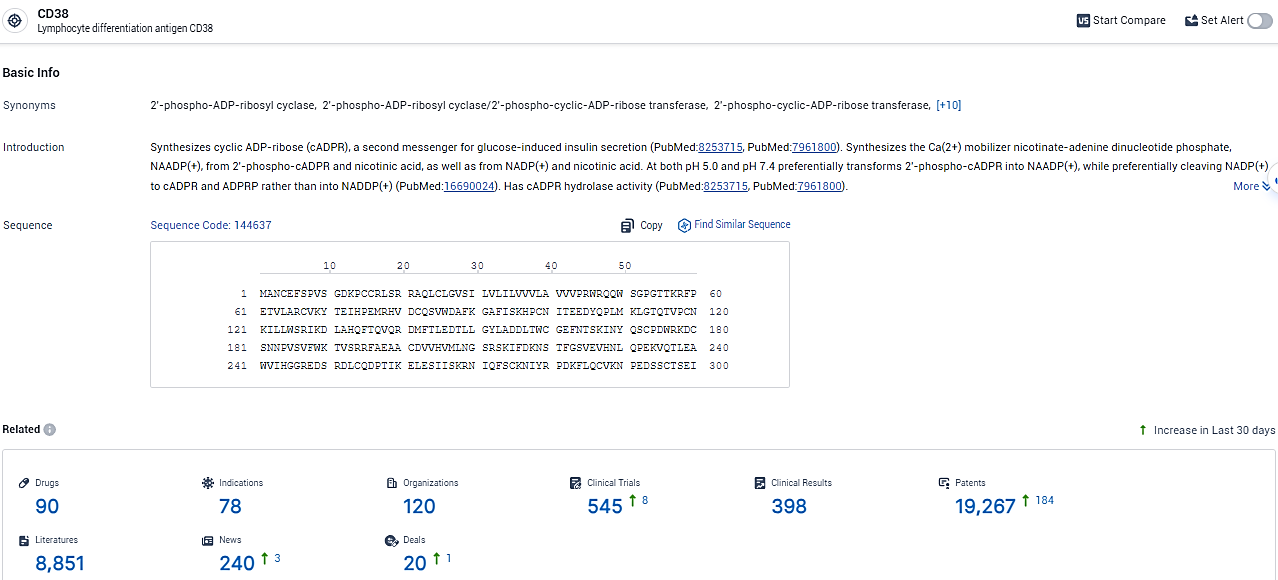
According to the data provided by the Synapse Database, As of February 7, 2024, there are 90 investigational drugs for the CD38 target, including 78 indications, 120 R&D institutions involved, with related clinical trials reaching 545, and as many as 19267 patents.
daratumumab is a monoclonal antibody drug that targets CD38 and has been approved for use in various therapeutic areas. It has shown efficacy in treating multiple indications and has undergone rigorous regulatory processes. DARZALEX FASPRO® (daratumumab and hyaluronidase-fihj) received U.S. FDA approval in May 2020 and is approved for eight indications in multiple myeloma, three of which are for frontline treatment in newly diagnosed patients who are transplant eligible or ineligible.