US and Japan Approve BMS's Breyanzi Filings for Recurrent Lymphomas
Bristol Myers Squibb has confirmed that both the FDA in the United States and the Japanese Ministry of Health, Labour and Welfare have sanctioned a trio of submissions regarding their therapeutic product, Breyanzi® (lisocabtagene maraleucel). Within the United States, the Biologics License Applications, submitted as supplemental by the firm, have been endorsed by the FDA to permit the usage expansion of Breyanzi. This expansion encompasses the management of relapsed or refractory follicular lymphoma in adults, as well as cases of relapsed or refractory mantle cell lymphoma following therapy with a Bruton tyrosine kinase (BTK) inhibitor.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.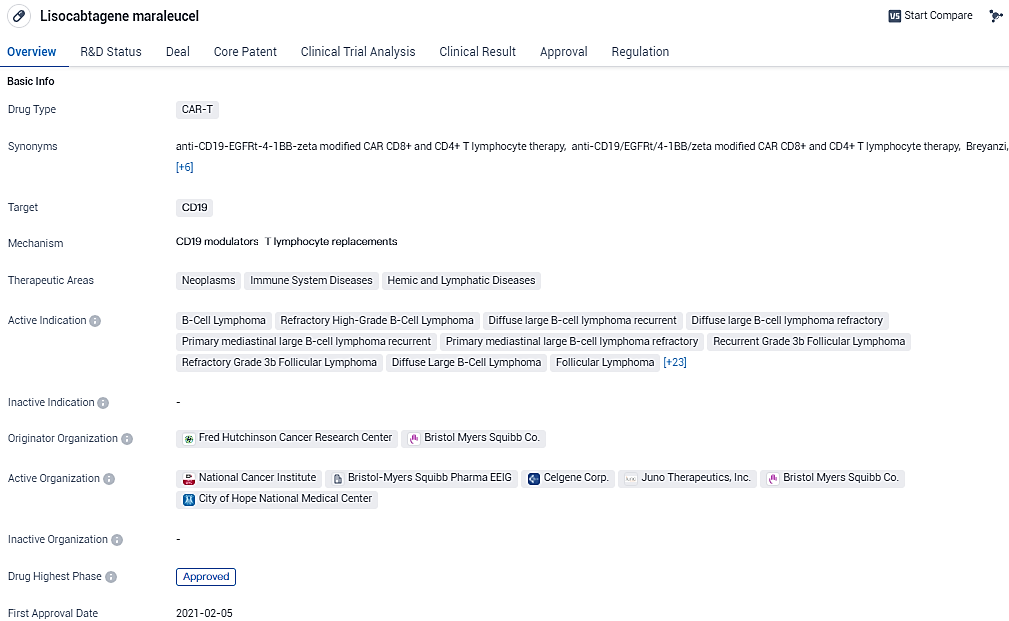
The U.S. Food and Drug Administration (FDA) has conferred Priority Review status on both submissions and has established a deadline under the Prescription Drug User Fee Act for Breyanzi, aiming for a decision by May 23, 2024, for its use in refractory or recurrent follicular lymphoma (FL) and by May 31, 2024, for its application in refractory or recurrent mantle cell lymphoma (MCL). Similarly, the Ministry of Health, Labour, and Welfare (MHLW) in Japan has approved the supplemental application by Bristol Myers Squibb to provide Breyanzi as a therapy for refractory or recurrent FL.
Anne Kerber, M.D., the senior vice president in charge of Hematology, Oncology, and Cell Therapy Late Clinical Development at Bristol Myers Squibb, remarked, “For individuals contending with FL and MCL, an ongoing pattern of disease remission followed by rebound is common, and our focus remains on advancing novel therapeutic options for these patients.” Kerber further stated, “Breyanzi is designed to elicit lasting responses, and the approvals of our submissions in both the United States and Japan underscore our pledge to extend the reach of our leading-edge CAR T cell therapies to the widest circle of candidates feasible.”
Moreover, a supplemental Biologics License Application (sBLA) for the administration of Breyanzi in adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) that have become resistant or did not respond following treatment with a prior Bruton's tyrosine kinase inhibitor (BTKi) and a B-cell lymphoma 2 (Bcl-2) inhibitor is presently in Priority Review at the FDA. The scheduled date for a decision is set at March 14, 2024.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
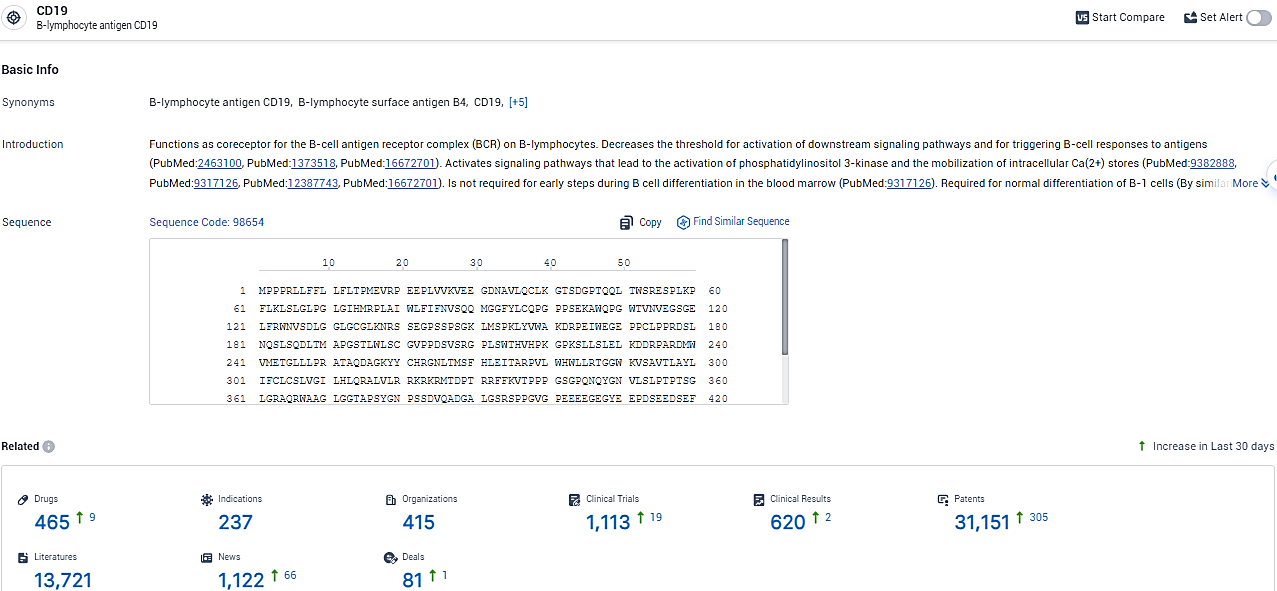
According to the data provided by the Synapse Database, As of February 7, 2024, there are 465 investigational drugs for the CD19 target, including 237 indications, 415 R&D institutions involved, with related clinical trials reaching 1113, and as many as 31151 patents.
Breyanzi is a CD19-directed CAR T cell therapy with a 4-1BB costimulatory domain, which enhances the expansion and persistence of the CAR T cells. Its first approval in the United States in February 2021, along with its priority review and orphan drug status, underscores its potential to improve outcomes for patients with lymphomas and leukemias.