Vertex Announces Promising Phase 3 Results of VX-548 for Acute Pain Management
Vertex Pharmaceuticals Incorporated has revealed encouraging outcomes from its Phase 3 studies focusing on VX-548, a targeted NaV1.8 inhibitor, for managing moderate-to-severe acute pain episodes. The comprehensive Phase 3 research consisted of two essential trials, both of which were randomized, double-blind, and placebo-controlled. The first trial took place post-abdominoplasty, while the second was conducted after bunionectomy procedures. Additionally, a distinct non-comparative study was implemented to evaluate the safety and efficacy of VX-548 across a diverse array of patients experiencing pain from various surgical and non-surgical origins.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.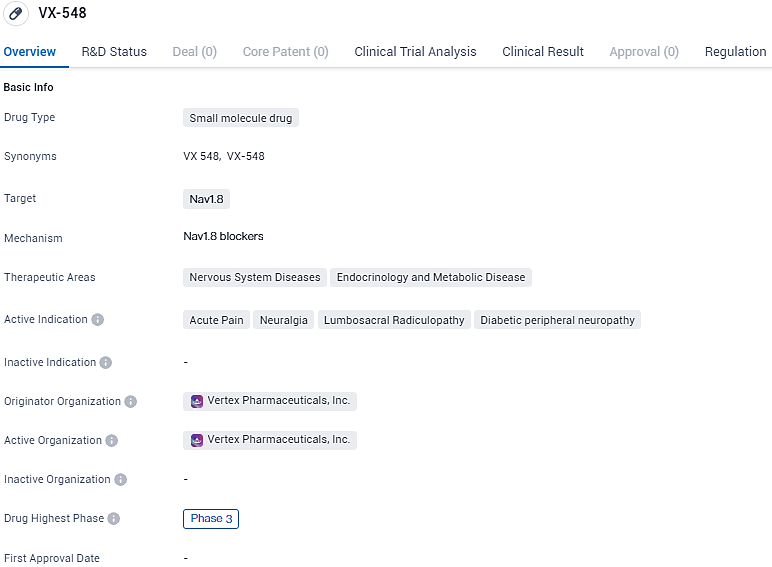
Administering VX-548 following procedures such as abdominoplasty or bunionectomy showed a notable and statistically significant enhancement in the main measure of the sum of pain intensity difference over a period of 48 hours when juxtaposed with a placebo. This also included a substantial decrease in pain levels at the 48-hour mark as per the Numeric Pain Rating Scale in each of the conducted study groups; specifically for bunionectomy: the adjusted least squares (LS) mean discrepancy in SPID48 between VX-548 and placebo was reported at 29.3.
The primary secondary aim tested the effectiveness of VX-548 against the combined medication of hydrocodone bitartrate/acetaminophen based on SPID48 after both abdominoplasty and bunionectomy operations. However, neither study achieved this particular secondary goal (abdominoplasty: LS mean variance for VX-548 as opposed to HB/APAP was 6.6 with a 95% Confidence Interval (CI): -5.4, 18.7; P=0.2781; bunionectomy: LS mean variance for VX-548 against HB/APAP marked at -20.2 with a 95% CI: -32.7, -7.7; P=0.0016)).
The secondary priority outcome for both studies was the duration until the patient experienced significant alleviation of pain, which was defined as a reduction of at least 2 points on the NPRS from the initial pain score, compared with the control group. In this regard, VX-548 facilitated faster advancement to noticeable pain alleviation than the placebo during both the abdominoplasty and bunionectomy research phases.
Additional secondary outcomes observed across both studies mirrored the outcomes of the primary goal.Lastly, a Phase 3 open-label study assessed the safety and efficacy of VX-548 used for a maximum of two weeks in a diverse spectrum of other acute pain scenarios, both surgical and nonsurgical. The results indicated a promising safety profile and acceptable tolerability, alongside positive effectiveness as indicated by a Patient Global Assessment at the conclusion of the therapy period.
Vertex's CEO and President, Dr. Reshma Kewalramani, expressed strong satisfaction with the pivotal results from VX-548's critical trials, highlighting the unique and consistent blend of safety and effectiveness the drug exhibited across varied acute pain situations. She emphasized the potential of VX-548 to address the need for a pain management option that straddles the divide between non-opioid medications, known for their safety but lesser effect, and opioids that are efficacious but come with well-documented issues, including the risk of addiction.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!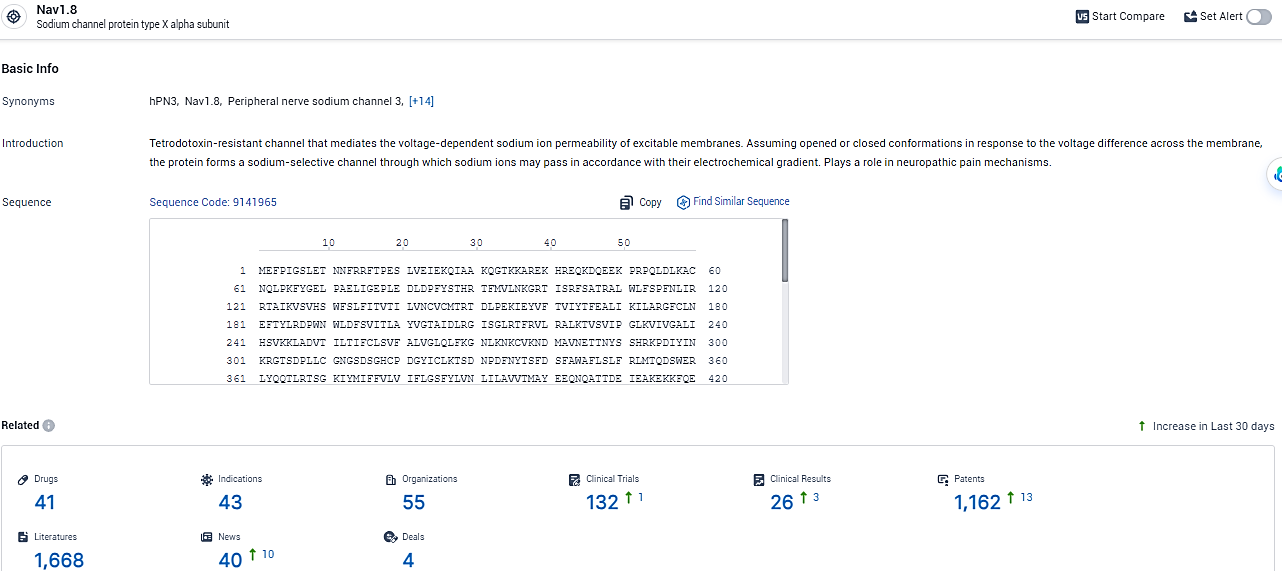
According to the data provided by the Synapse Database, As of February 6, 2024, there are 41 investigational drugs for the NaV1.8 target, including 43 indications, 55 R&D institutions involved, with related clinical trials reaching 132, and as many as 1162 patents.
VX-548 targets Nav1.8 and is indicated for the treatment of various conditions related to acute pain and neuropathy. With its current status in Phase 3 clinical development and regulatory designations, VX-548 shows promise as a potential therapeutic option for patients suffering from these conditions.




