Jeune Aesthetics Reports Positive Phase 1 Results for KB301 in Reducing Eye and Décolletage Wrinkles
Jeune Aesthetics, Inc. ("Jeune"), a fully integrated entity under Krystal Biotech, Inc.'s ("Krystal") (NASDAQ: KRYS) umbrella, leverages Krystal's clinically proven gene-delivery system to fundamentally tackle and reverse the biological mechanisms underlying aging skin. Jeune proudly unveiled favorable interim safety and effectiveness outcomes from Cohorts 3 and 4 of the PEARL-1 Phase 1 study. This study explores KB301, an investigational aesthetic therapy aimed at delivering the COL3A1 transgene to elevate type III collagen ("COL3") concentrations in the skin. Cohort 3 focuses on alleviating resting lateral canthal lines, while Cohort 4 targets the reduction of dynamic wrinkles on the décolleté.
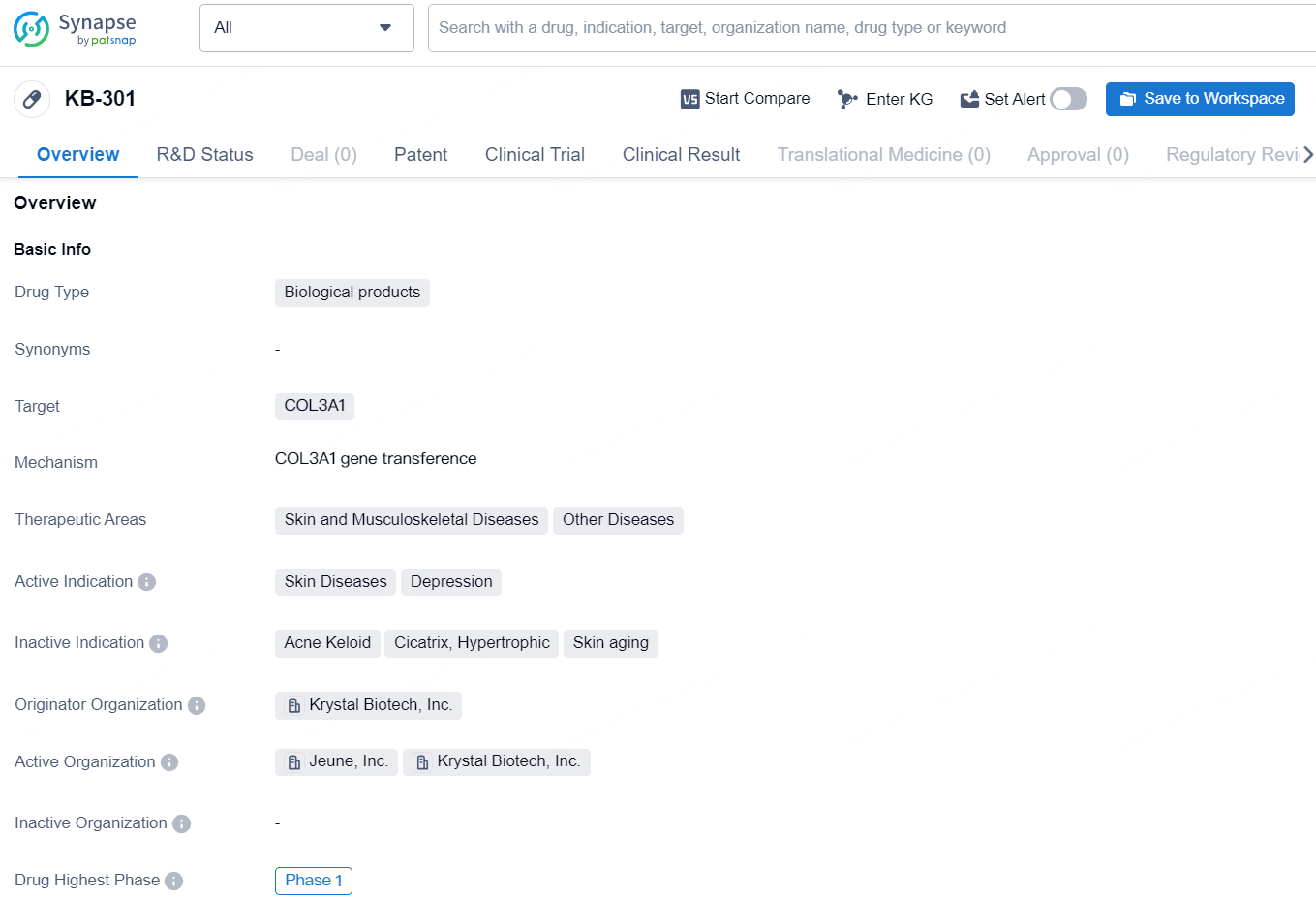
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The current arsenal of injectable aesthetic treatments is confined to toxins and fillers, empowering us to manipulate but not rejuvenate aging skin," remarked Dr. Steve G. Yoelin, a key investigator in the PEARL-1 study. "KB301, with its distinctive mode of action and promising initial efficacy findings, ignites my enthusiasm for its potential to revolutionize the medical aesthetics landscape and cater to the escalating need for treatments that fundamentally replenish skin or defer aging signs."
Both investigators and participants concurred in reporting substantial and enduring enhancements in skin aesthetics, as evaluated by the Global Aesthetic Improvement Scale (GAIS), across both the décolleté and lateral canthal areas. Furthermore, heightened satisfaction with wrinkle appearance was documented through the Subject Satisfaction Questionnaire (SSQ).
Efficacy Highlights for Dynamic Wrinkles on the Décolleté
A cohort of 20 subjects was enrolled, with two withdrawing prior to completing KB301 treatments. The remaining 18 subjects underwent aesthetic assessment up to two months post-injection in the décolleté region. Key findings include:
Clinically significant wrinkle improvement, as per GAIS, was reported by investigators at both one and two months post-treatment:
Two months: 94% of subjects exhibited at least a one-point improvement, with 28% achieving the maximum two-point improvement.
One month: 83% of subjects showed at least a one-point improvement, and 28% reached a two-point improvement.
Subjects' self-reported wrinkle improvements, also using GAIS, intensified from the first to the second follow-up month:
Two months: 89% reported at least a one-point improvement, and 39% noted a two-point improvement.
One month: 61% reported at least a one-point improvement, with 28% reporting a two-point improvement.
SSQ revealed that 94% of subjects expressed heightened satisfaction with their wrinkle appearance two months post-treatment.
Moreover, notable improvements were observed in additional skin attributes, such as crepiness, hydration, and radiance, as evaluated by GAIS. Investigators documented improvements of one point or more in 89%, 94%, and 94% of subjects, respectively, two months after treatment.
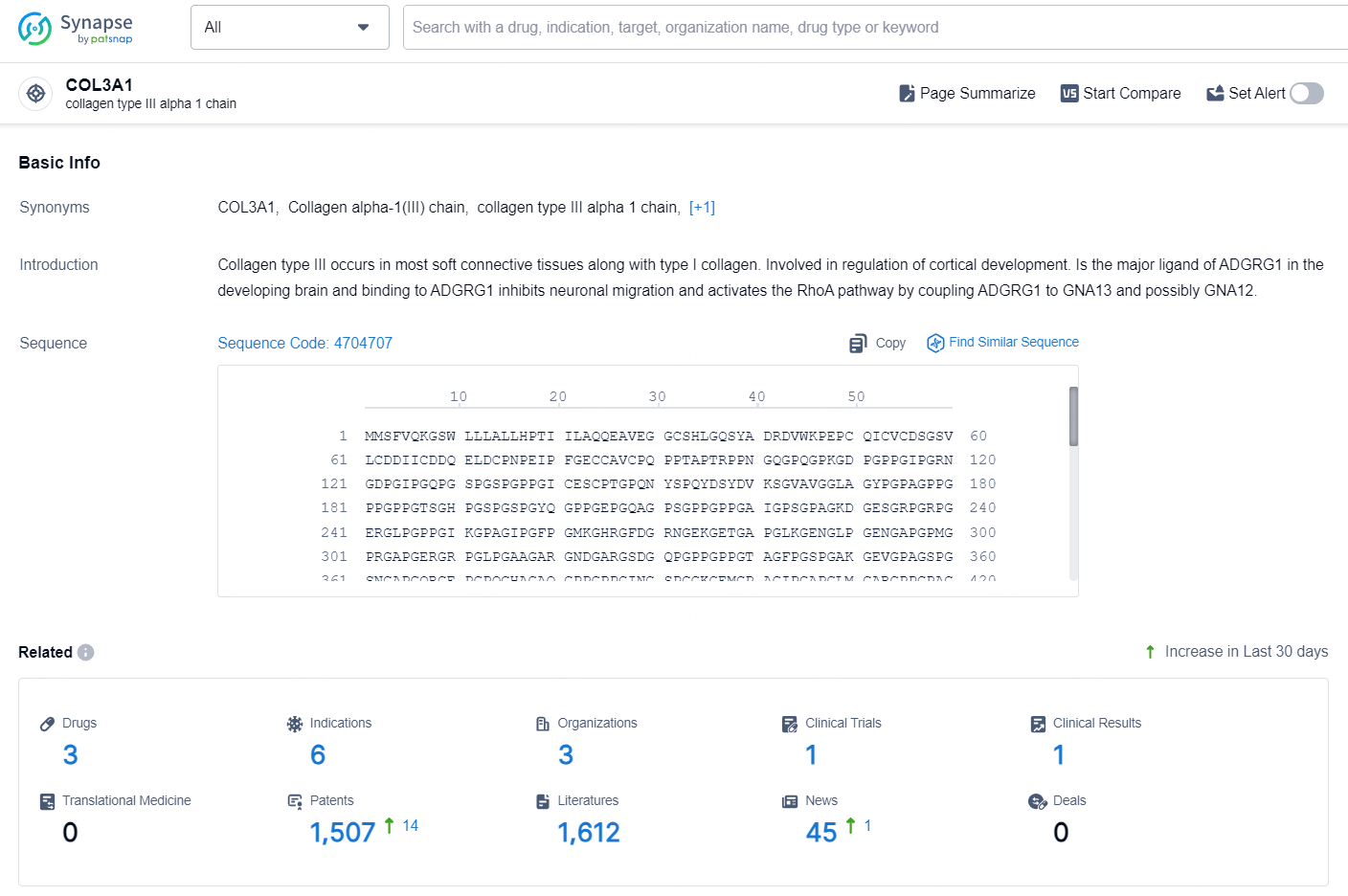
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 3 investigational drugs for the COL3A1 target, including 6 indications, 3 R&D institutions involved, with related clinical trial reaching 1, and as many as 1507 patents.
KB301 is an investigational aesthetic therapy employing Krystal’s novel replication-defective, non-integrating HSV-1-based vector to deliver two copies of the COL3A1 transgene and increase COL3 levels in skin to address signs of skin aging associated with declining collagen levels and damage of the skin’s extracellular matrix. KB301 is formulated as a solution for direct intradermal injection to aesthetic priority areas.