KaliVir Immunotherapeutics Begins Phase 1/1b Trial of VET3-TGI in Advanced Solid Tumors
KaliVir Immunotherapeutics, Inc., a biotechnology firm at the clinical stage focusing on innovative, multi-action oncolytic viral immunotherapy solutions, revealed that it has administered the initial dose to a patient in their STEALTH-001 study. This study represents a Phase 1/1b clinical trial for VET3-TGI, aimed at individuals suffering from advanced solid tumors that are incurable. VET3-TGI is a groundbreaking oncolytic immunotherapy, engineered to specifically target and destroy tumor cells, while also delivering an immune-boosting transgene payload made up of interleukin-12 and a TGFbeta inhibitor.
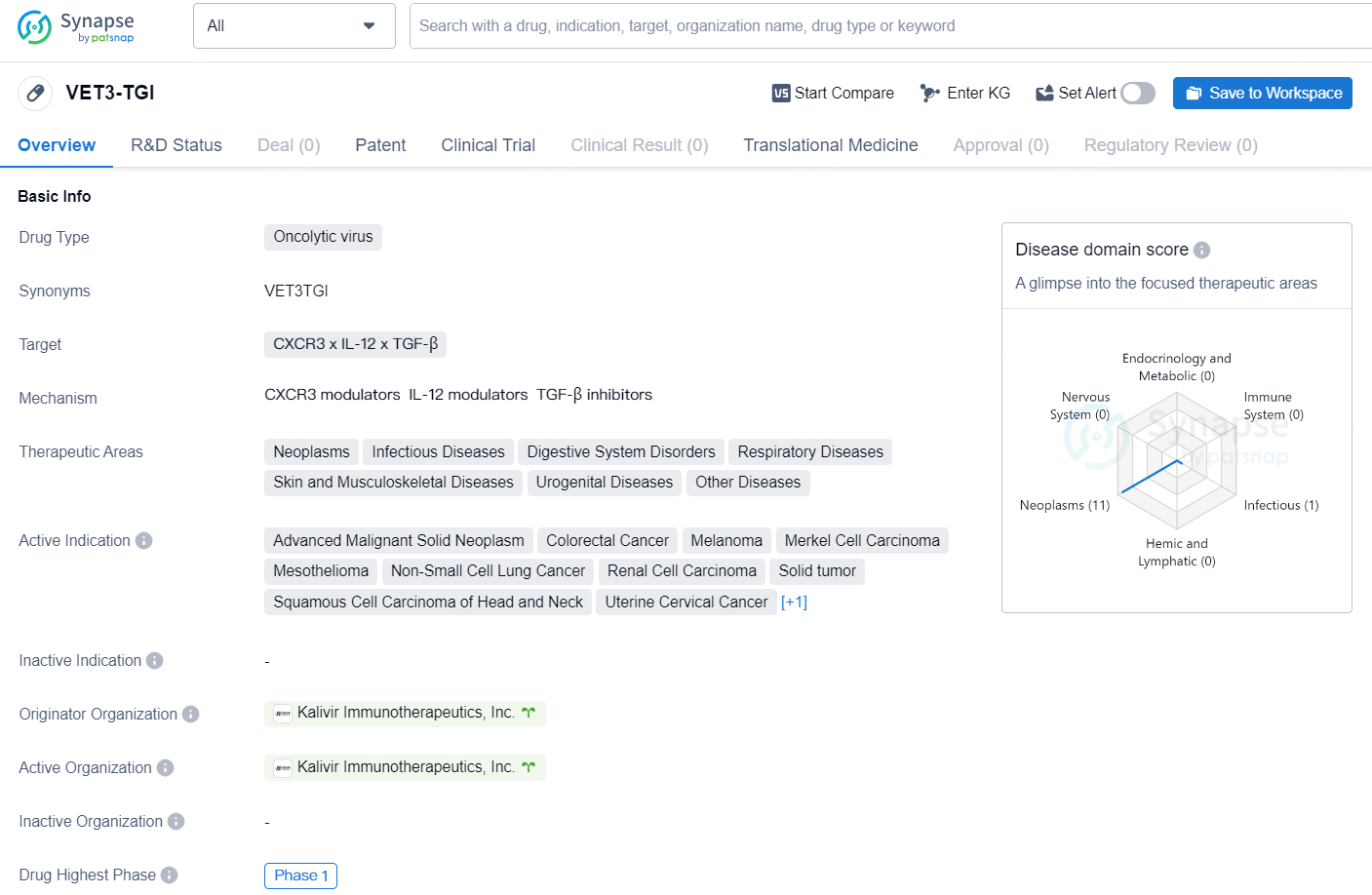
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
James Burke, M.D., the Chief Medical Officer at KaliVir Immunotherapeutics, emphasized the importance of administering the first patient dose in the STEALTH-001 study, calling it a major milestone for both the company and their VET3-TGI program. This platform is uniquely designed to target tumors selectively, even when anti-viral immunity is present, and offers potential in delivering powerful immune stimulatory agents intravenously to patients with advanced solid tumors. The study will examine VET3-TGI as both a standalone treatment and in combination with checkpoint inhibitors.
Regarding the STEALTH-001 Clinical Trial
The STEALTH-001 trial (ClinicalTrials.gov No. NCT06444815) is designed to explore dose escalation and expansion, administering VET3-TGI either directly into tumors via injection or through intravenous delivery. The purpose of dose escalation is to determine the maximum tolerated dosage of VET3-TGI when administered in these various ways, including its use alongside checkpoint blockade. After identifying the maximum tolerated dose for each scenario, the study will expand to further assess the safety and efficacy of VET3-TGI. Participants are patients with histologically confirmed advanced, inoperable, or metastatic solid tumors.
Jorge Nieva, M.D., an Associate Professor of Clinical Medicine at the Keck School of Medicine, USC, as well as the Section Head for Lung and Head/Neck Tumors at the Norris Comprehensive Cancer Center and member of KaliVir’s Medical Advisory Board, expressed optimism about VET3-TGI's potential to change the treatment landscape for advanced tumors. He highlighted the milestone's significance and expressed eagerness to observe the progress of VET3-TGI as both a monotherapy and when used with checkpoint inhibitor therapy.
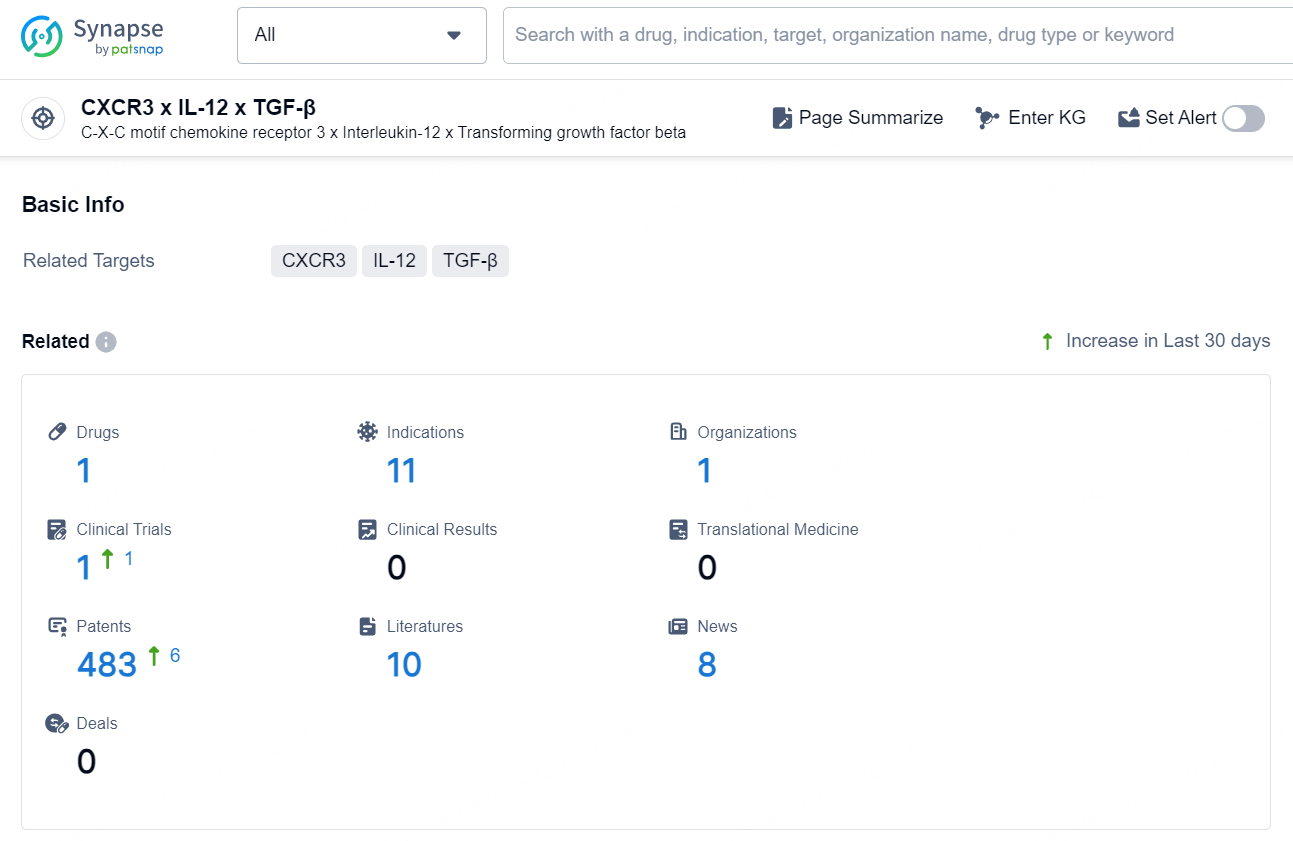
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 1 investigational drug for the CXCR3, IL-12, and TGF-β targets, including 11 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 483 patents.
VET3-TGI is an oncolytic virus drug that is being developed by Kalivir Immunotherapeutics, Inc. The drug targets a combination of CXCR3, IL-12, and TGF-β, and has a wide range of therapeutic areas, including neoplasms, infectious diseases, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.