Ketanserin: Detailed Review of its Transformative R&D Success
Ketanserin's R&D Progress
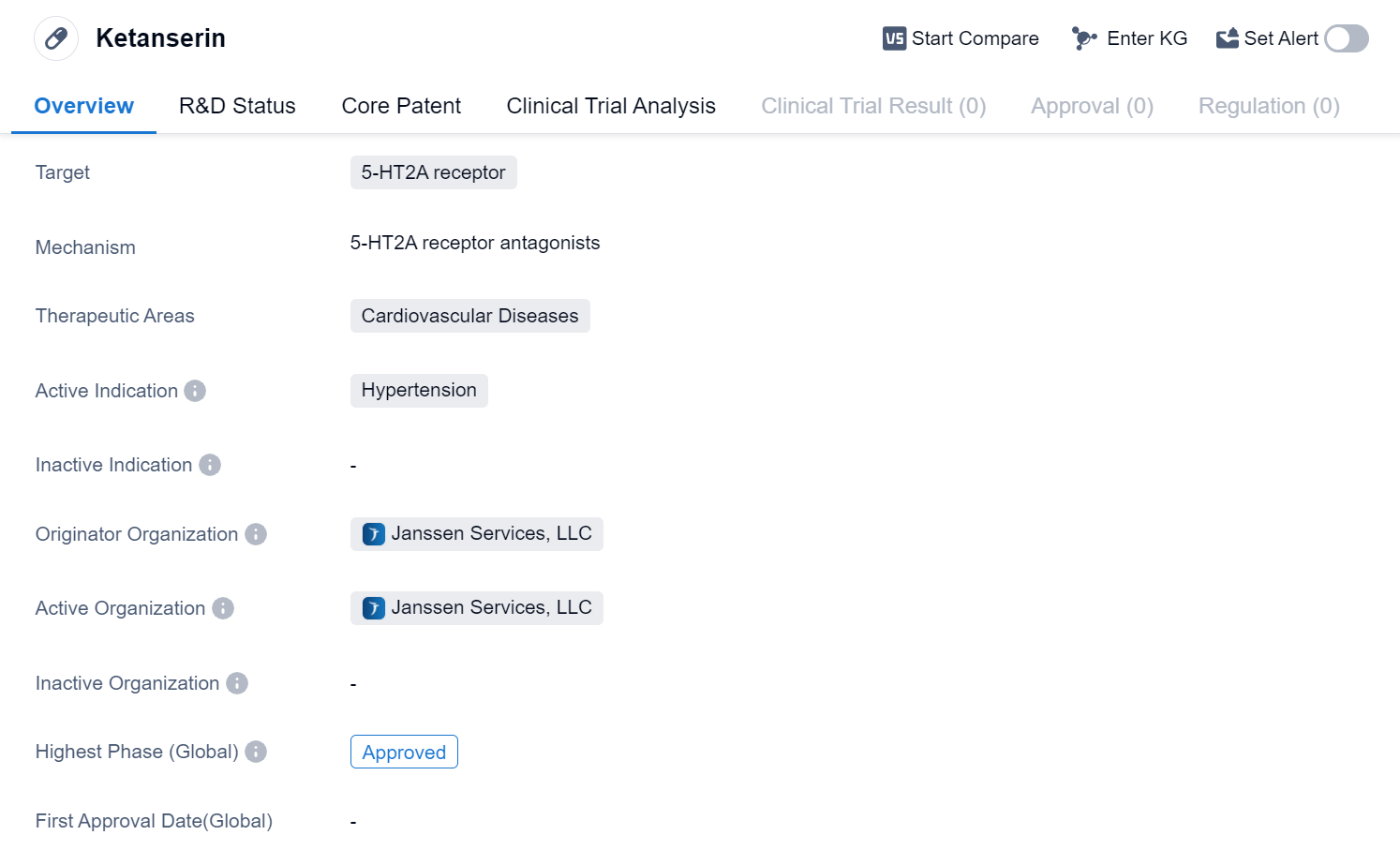
Ketanserin is a small molecule drug that targets the 5-HT2A receptor. It is primarily used in the treatment of cardiovascular diseases, specifically hypertension. The drug has been approved for use and is currently in the highest phase of development globally.
Ketanserin is developed by Janssen Services, LLC, an originator organization in the pharmaceutical industry. The drug's mechanism of action involves blocking the 5-HT2A receptor, which is responsible for regulating blood pressure and vascular tone. By inhibiting this receptor, Ketanserin helps to lower blood pressure and reduce the risk of cardiovascular complications associated with hypertension.
Hypertension, or high blood pressure, is a common condition that affects a significant portion of the global population. It is a major risk factor for cardiovascular diseases such as heart attacks and strokes. Managing hypertension is crucial in preventing these serious health complications, and medications like Ketanserin play a vital role in achieving optimal blood pressure control.
It has successfully completed the necessary clinical trials and met the regulatory requirements for market authorization. This approval allows healthcare professionals to prescribe Ketanserin to patients with hypertension, providing them with an effective treatment option to manage their condition.
As a small molecule drug, Ketanserin is likely to be available in oral dosage forms, such as tablets or capsules, making it convenient for patients to take. The drug's specific targeting of the 5-HT2A receptor suggests that it may have a more focused therapeutic effect, potentially minimizing side effects compared to drugs that act on multiple receptors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ketanserin: 5-HT2A receptor antagonists
5-HT2A receptor antagonists are a class of drugs that specifically target and block the activity of the 5-HT2A receptors in the brain. These receptors are a subtype of serotonin receptors, which are involved in the regulation of various physiological and neurological processes. By blocking the 5-HT2A receptors, antagonists can modulate the effects of serotonin, a neurotransmitter that plays a key role in mood, cognition, and perception.
From a biomedical perspective, 5-HT2A receptor antagonists are primarily used in the treatment of psychiatric disorders such as depression, anxiety, and schizophrenia. By inhibiting the activity of these receptors, these drugs can help regulate serotonin levels and improve symptoms associated with these conditions. Additionally, some 5-HT2A receptor antagonists have also shown potential in the treatment of sleep disorders and substance abuse.
It is important to note that while 5-HT2A receptor antagonists can be beneficial in certain therapeutic contexts, they may also have side effects. These can include drowsiness, dizziness, gastrointestinal disturbances, and changes in blood pressure. Therefore, the use of these drugs should be carefully monitored and prescribed by healthcare professionals.
Drug Target R&D Trends for Ketanserin
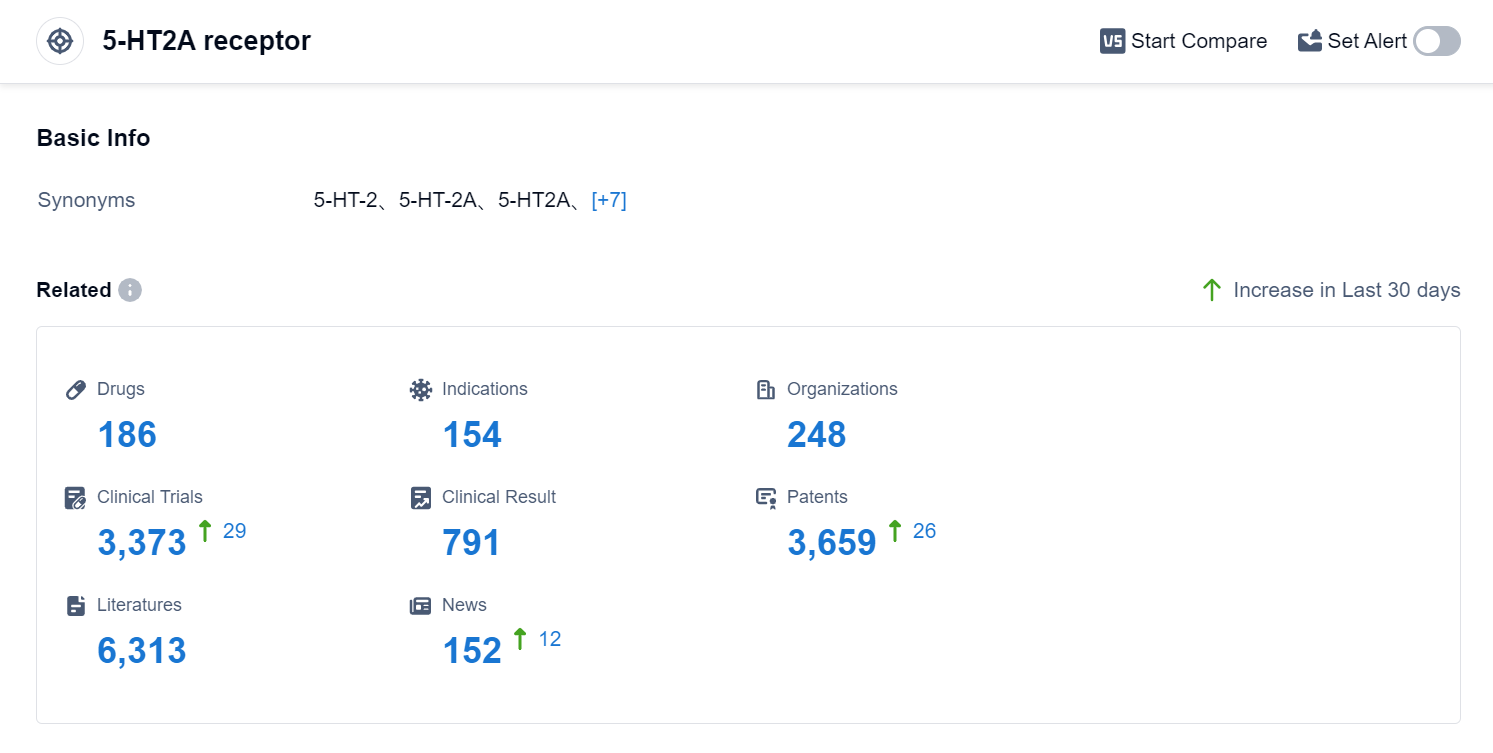
According to Patsnap Synapse, as of 1 Sep 2023, there are a total of 186 5-HT2A receptor drugs worldwide, from 248 organizations, covering 154 indications, and conducting 3373 clinical trials.
In conclusion, the target 5-HT2A receptor has attracted the attention of several pharmaceutical companies, with Sumitomo Chemical Co., Ltd., Novartis AG, Johnson & Johnson, Teva Pharmaceutical Industries Ltd., and Otsuka Holdings Co., Ltd. leading in terms of R&D progress. Drugs targeting the 5-HT2A receptor have been approved for indications such as schizophrenia, bipolar disorder, depressive disorder, and psychotic disorders. Small molecule drugs are progressing most rapidly, indicating intense competition in the market. The United States, Japan, and the European Union are the leading countries/locations in terms of drug development, with China also showing progress. Overall, the target 5-HT2A receptor presents a competitive landscape with potential for future development in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Conclusion
In summary, Ketanserin is a small molecule drug developed by Janssen Services, LLC, targeting the 5-HT2A receptor. It is approved for use in the treatment of hypertension, a common cardiovascular disease. Ketanserin's specific mechanism of action and its originator organization's reputation in the pharmaceutical industry make it a promising option for managing hypertension and reducing the risk of associated complications.