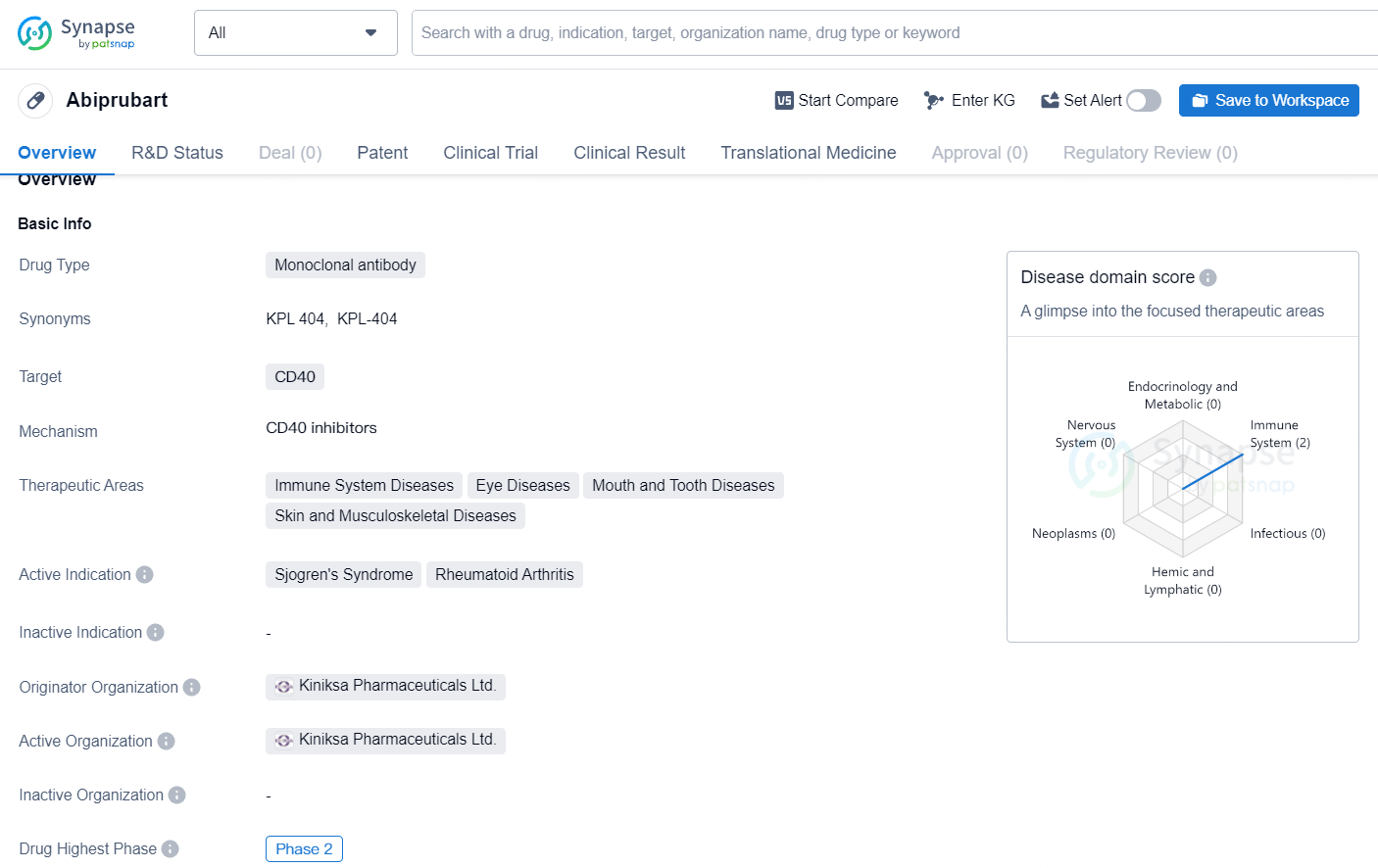
Kiniksa Pharmaceuticals Starts Phase 2b Trial for Abiprubart in Sjögren’s Disease
Kiniksa Pharmaceuticals International, plc, a biopharmaceutical company operating at the commercial stage and possessing a range of immune-modulating assets aimed at addressing various cardiovascular and autoimmune conditions, announced that it has begun enrolling participants for the Phase 2b clinical trial of abiprubart in Sjögren’s Disease. Abiprubart, an experimental humanized anti-CD40 monoclonal antibody, is designed to block the CD40-CD154 interaction.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Sjögren’s Disease is a chronic, debilitating autoimmune condition that currently has no FDA-approved treatments. Beginning the next stage of development for abiprubart in the context of Sjögren’s Disease is a critical advancement for patients,” stated Sanj K. Patel, Chairman and CEO of Kiniksa.
“Our Phase 2b clinical trial builds on existing mechanistic proof-of-concept data as well as insights gained from our previous clinical trials. Furthermore, we believe that abiprubart, administered conveniently via subcutaneous injection, has the potential to meet unmet medical needs in this area. Notably, our ongoing operational plan includes the clinical progress of abiprubart for Sjögren’s Disease, and the company anticipates maintaining positive cash flow on an annual basis,” added Patel.
The double-blind, placebo-controlled Phase 2b trial is structured to assess the efficacy of chronic subcutaneous administration of abiprubart in Sjögren’s Disease patients.
This part of the trial will randomize approximately 201 patients into three groups (1:1:1 ratio) to receive either abiprubart 400 mg biweekly, 400 mg monthly, or a placebo over 24 weeks. The primary outcome measure is the change in the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) from baseline compared to placebo at Week 24. Following this period, patients will transition into a long-term extension where all groups will receive active treatment for another 24 weeks.
Abiprubart, an investigational humanized monoclonal antibody, targets and binds to CD40, aiming to inhibit the CD40-CD154 interaction. This interaction is crucial for T-cell co-stimulation, B-cell maturation, immunoglobulin class switching, and Type 1 immune responses. Kiniksa posits that blocking the CD40-CD154 co-stimulatory interaction presents a promising strategy for tackling various autoimmune diseases.
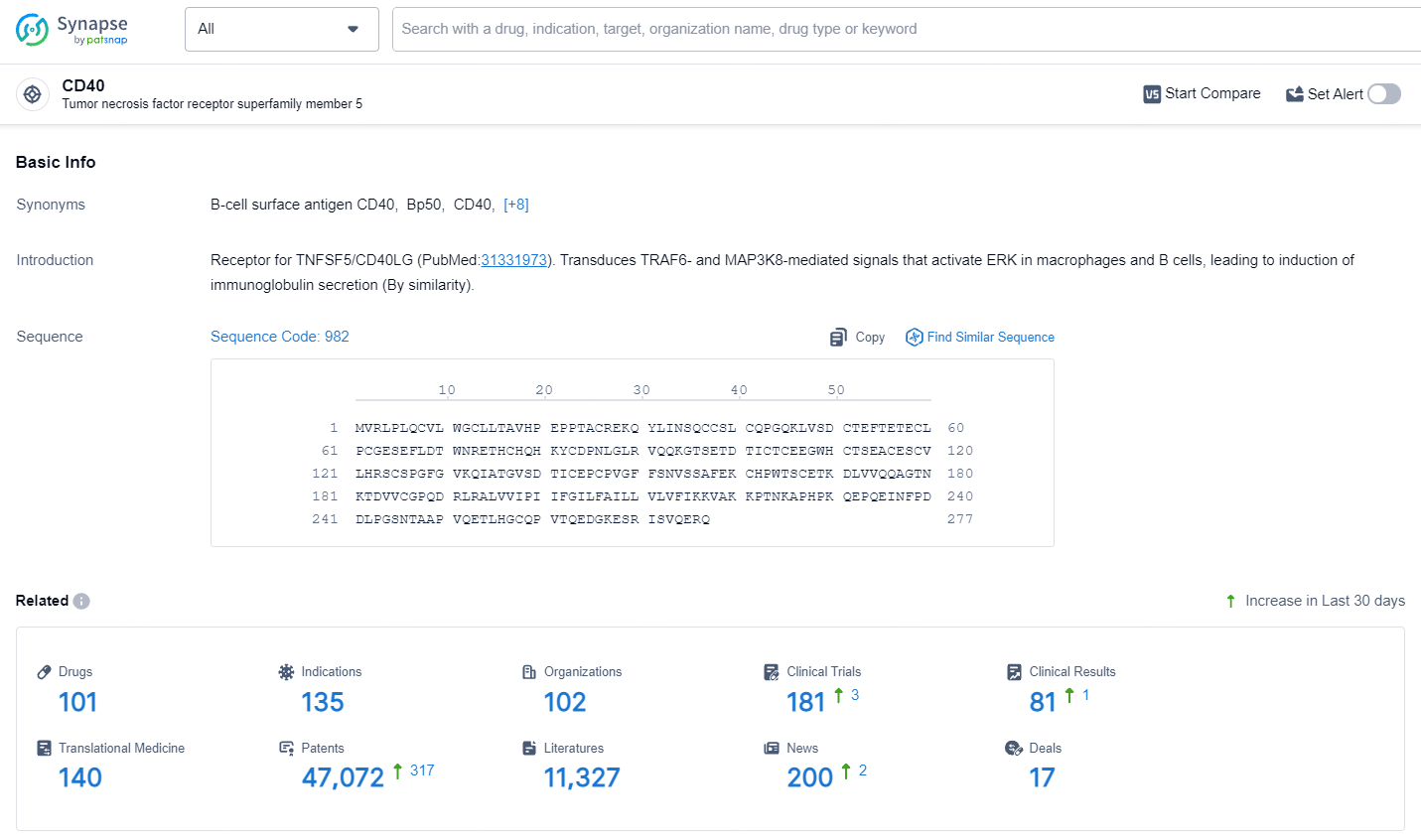
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 101 investigational drugs for the CD40 target, including 135 indications, 102 R&D institutions involved, with related clinical trials reaching 181, and as many as 47072 patents.
Abiprubart represents a novel approach to targeting CD40 in the treatment of rheumatoid arthritis and potentially other related conditions. Its advancement to Phase 2 underscores the interest and investment in further developing this monoclonal antibody for the benefit of patients with immune system, skin, and musculoskeletal diseases.