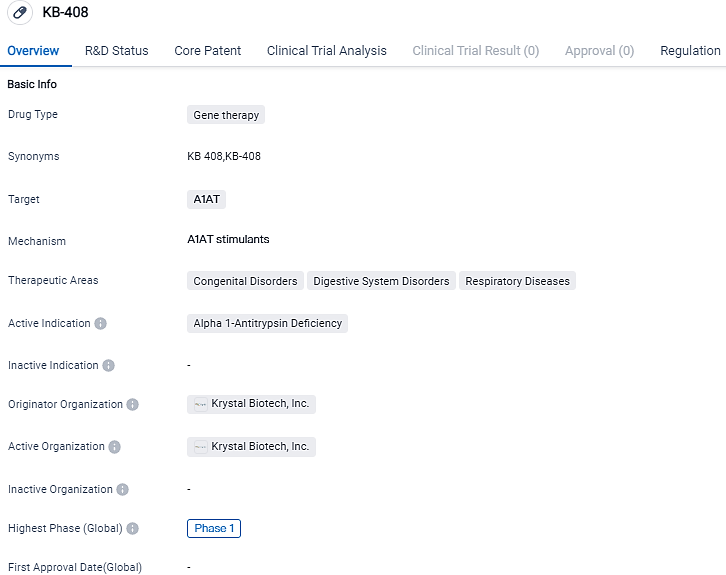
Krystal Biotech reports FDA approval of the investigational new drug application for KB408 to treat Type 1 Alpha-1 Antitrypsin Deficiency
Krystal Biotech, Inc., a biotech firm aiming to uncover, nurture, and launch genetic treatments for ailments with significant unaddressed healthcare necessities, publicized that the U.S. FDA has given the green light for the Investigational New Drug application for KB408 to manage alpha-1 antitrypsin deficiency.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
KB408 is an altered, non-replicating HSV-1 derivative vector that doesn't integrate, which carries a pair of full-length copies of the Serpin family A member 1 gene to enable the production of alpha-1 antitrypsin (AAT). It is designed to be delivered to the lung's respiratory cells through inhaled nebulization.
"We are thrilled to progress KB408, our experimental gene therapy for patients suffering from alpha-1 antitrypsin deficiency, in our clinical Serpentine-1 trial," expressed Hubert Chen, M.D., the Chief of Clinical Development at Krystal Biotech. Dr. Chen added, "The acceptance of this IND is a significant step forward in our efforts to tackle a severe lung disease with few available treatments, and it also provides an opportunity to showcase the potential of our platform to repeatedly deliver genes to the lung's epithelial cells."
On August 15, the corporation sent an IND application to the FDA to gain authorization to commence a Phase 1 clinical trial of KB408. By the conclusion of the 30-day evaluation period, the corporation had received FDA confirmation that the IND had been approved. The first Phase 1 clinical trial patient for KB408 is expected to be administered in Q1 2024. The FDA granted orphan-drug status for KB408 for AATD therapy on September 5th.
The Phase 1 clinical trial is an open-label, single dose escalation Phase 1 study involving adult AATD patients with a PI*ZZ genotype. Three different dosing stages for KB408 will be evaluated with three patients in each group to assess the drug's safety, tolerability, and efficacy.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
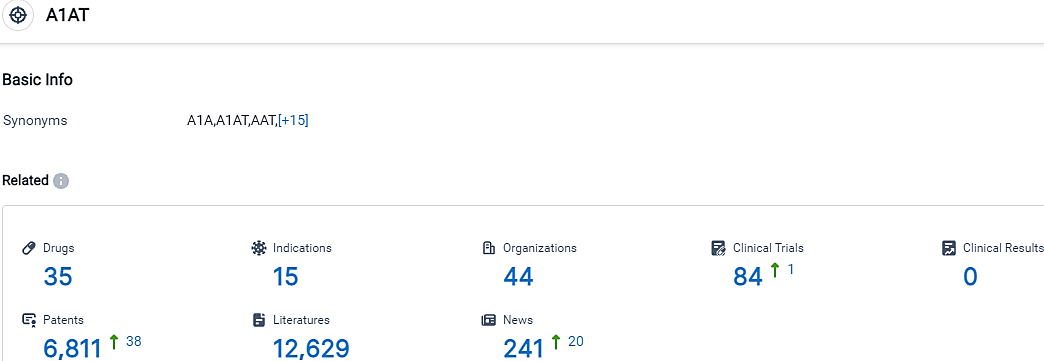
According to the data provided by the Synapse Database, As of September 28, 2023, there are 35 investigational drugs for the A1AT target, including 15 indications,44 R&D institutions involved, with related clinical trials reaching 84,and as many as 6811 patents.
KB-408, a novel pharmaceutical formulation to be inhaled, hails from the company's innovative replication-inoperative, non-integrating HSV-1-based vector, developed to transport dual copies of the encoded SERPINA1 transgene, which is linked with human alpha-1 antitrypsin protein, aimed for AATD treatment. With an emphasis on inborn anomalies, gastrointestinal disorders, and respiratory illnesses, KB-408 presents potential benefits in treating a spectrum of diseases.