FDA Approves Vivani Medical's NPM-119 GLP-1 Subdermal Implant and Lifts Clinical Hold
Vivani Medical, Inc., a pioneering biopharmaceutical company focused on creating new, extended-release drug implants, declared that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug Application and removed the clinical hold on NPM-119. This clearance permits the commencement of LIBERATE-1™, a Phase 1 clinical trial designed to evaluate the safety, tolerability, and pharmacokinetics of NPM-119 (exenatide). The product is the Company’s compact, six-month GLP-1 implant being developed for the treatment of type 2 diabetes.
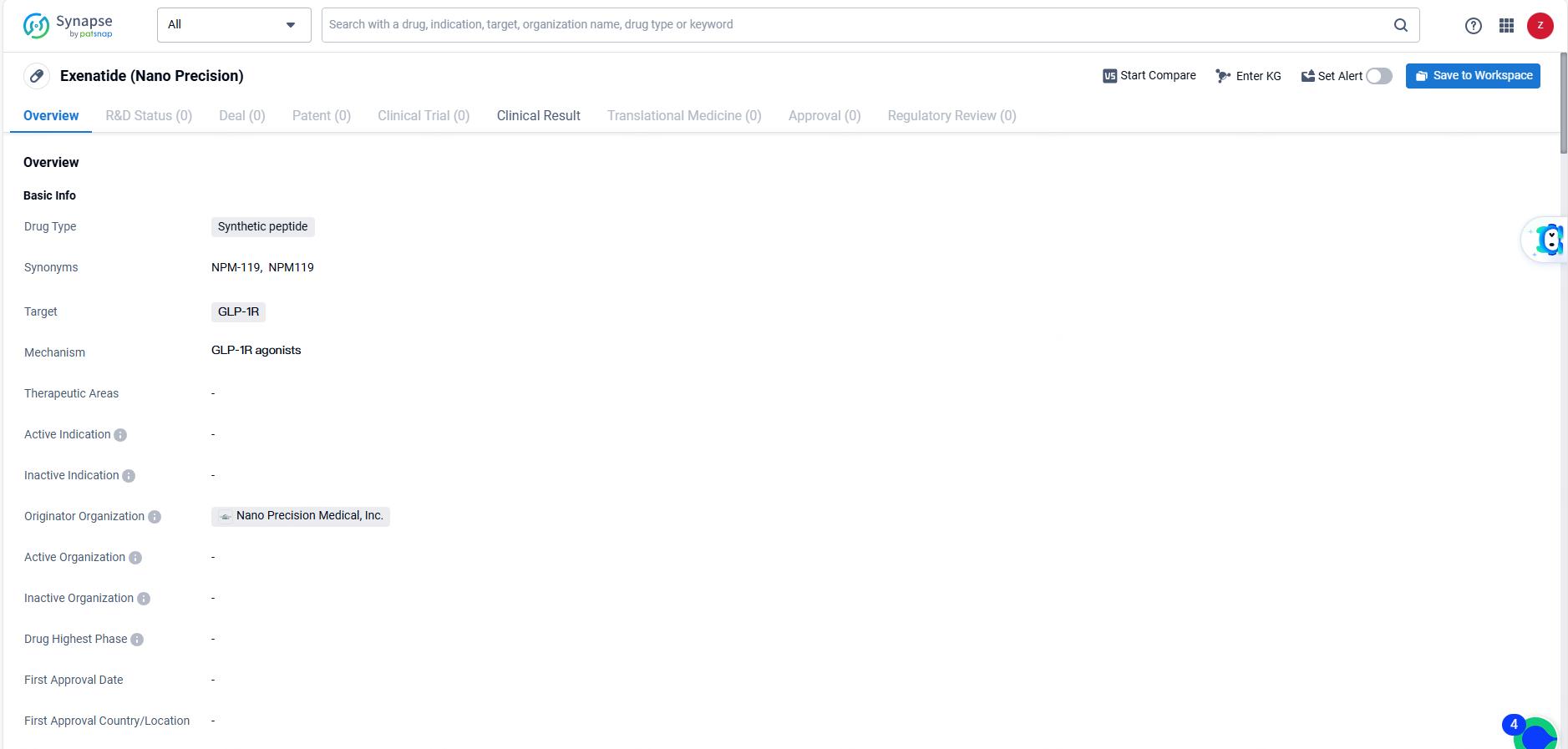
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Vivani is reaching an important milestone as we evolve into a clinical-stage enterprise with a drug candidate that shows promise in addressing the issue of medication non-adherence, which impacts nearly 50% of individuals with type 2 diabetes. LIBERATE-1 stands as our inaugural human study of NPM-119 for type 2 diabetes patients and marks the initial clinical trial utilizing our pioneering NanoPortal™ implant technology in human subjects," stated Adam Mendelsohn, Ph.D., President and CEO of Vivani.
"This groundbreaking research aims to assess how the encouraging preclinical pharmacokinetic profile of NPM-119 compares in humans against the currently available once-weekly exenatide, Bydureon BCise," added Adam Mendelsohn.
LIBERATE-1 is a 12-week, randomized study that will examine the safety, tolerability, and comprehensive pharmacokinetic profile of NPM-119 in type 2 diabetes patients. Participants who had previously received GLP-1 therapy, which will be discontinued, will be enrolled and assigned either NPM-119 or the active comparator.
Vivani is continuing to advance its innovative, differentiated drug implants pipeline, utilizing its proprietary NanoPortal™ subdermal implant technology aimed at ensuring medication adherence and enhancing patient outcomes in the management of chronic diseases like chronic weight management and type 2 diabetes.
Beyond NPM-119 for type 2 diabetes, Vivani is also progressing in the development of NPM-115 (a high-dose exenatide implant) and NPM-139 (a semaglutide implant), both of which are prospective treatments for chronic weight management. Additionally, NPM-139 presents the possibility of an annual treatment regimen."
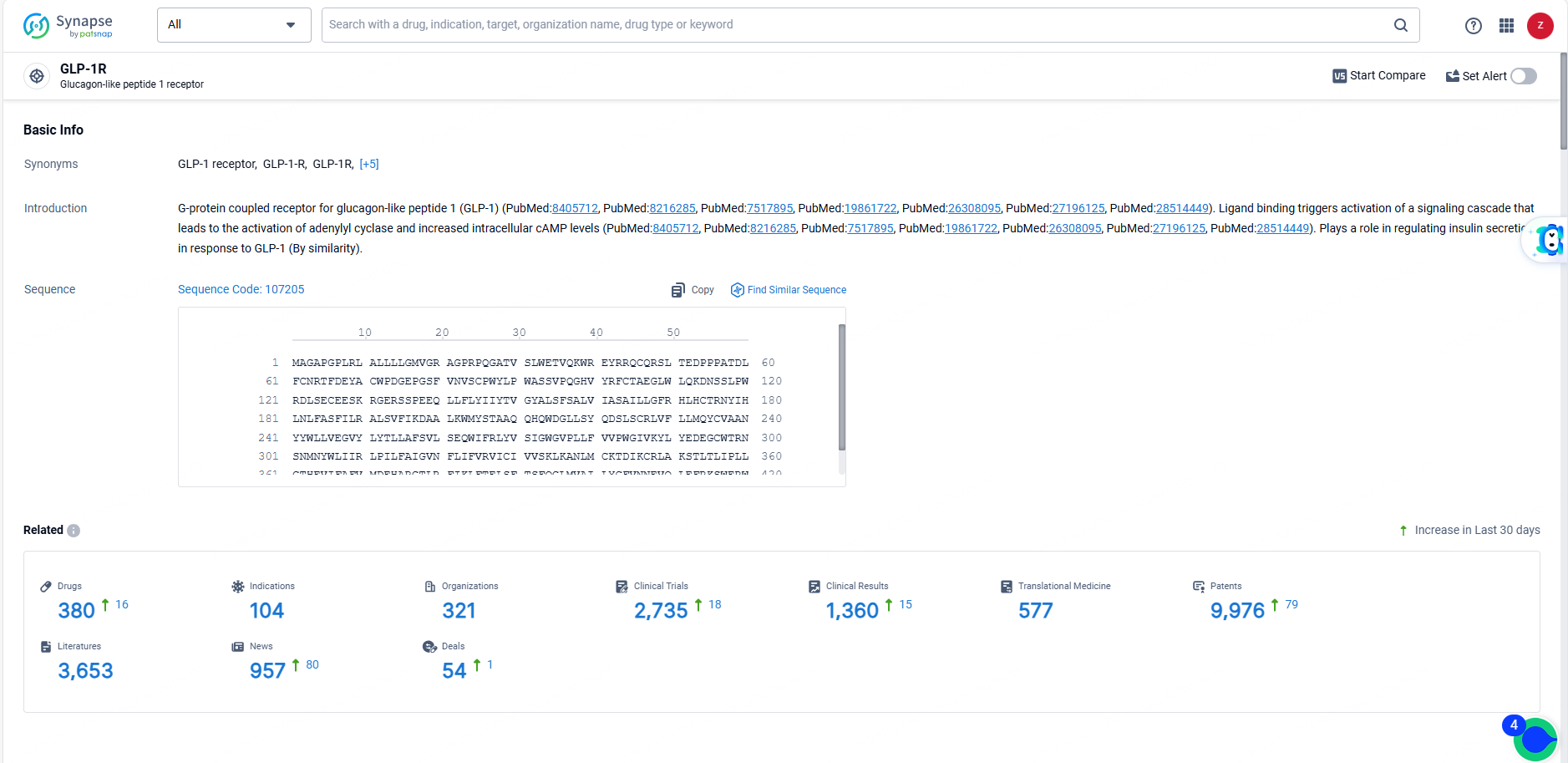
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 14, 2024, there are 380 investigational drugs for the GLP-1R targets, including 104 indications, 321 R&D institutions involved, with related clinical trials reaching 2735, and as many as 9976 patents.
Exenatide is a synthetic peptide drug developed by Nano Precision Medical, Inc. that targets the GLP-1R. As a pharmaceutical organization specializing in biomedicine, Nano Precision Medical, Inc. has been at the forefront of developing innovative pharmaceutical products in this field.