A Comprehensive Review of Melarsoprol's R&D Innovations and Drug Target Mechanism
Melarsoprol's R&D Progress
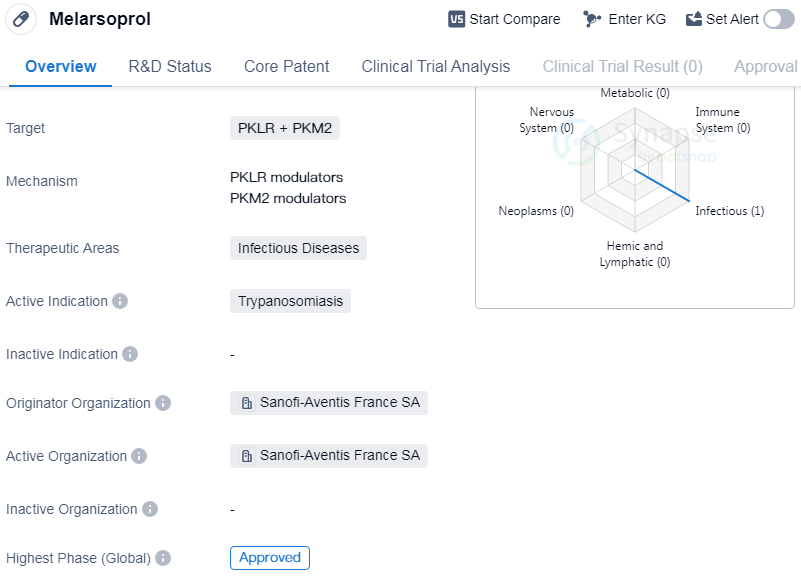
Melarsoprol is a small molecule drug that is primarily used in the treatment of trypanosomiasis, a parasitic infection caused by the protozoan parasite trypanosoma brucei. It was first approved for use in France in May 1997 and is currently in the highest phase of development, which is approved for global use.
The drug targets two enzymes, PKLR and PKM2, which are believed to play a crucial role in the survival and replication of the parasite. By inhibiting these enzymes, Melarsoprol disrupts the metabolic processes of the parasite, leading to its death.
Melarsoprol falls under the therapeutic area of infectious diseases, specifically targeting trypanosomiasis. This disease is prevalent in sub-Saharan Africa and is transmitted to humans through the bite of infected tsetse flies. If left untreated, it can lead to severe neurological complications and even death.
The originator organization of Melarsoprol is Sanofi-Aventis France SA, a multinational pharmaceutical company based in France. They have been responsible for the development and commercialization of the drug.
The approval of Melarsoprol in 1997 marked a significant milestone in the treatment of trypanosomiasis. Prior to its approval, treatment options for this disease were limited and often ineffective. Melarsoprol has shown promising results in clinical trials, demonstrating its efficacy in treating the infection and improving patient outcomes.
Since its approval, Melarsoprol has been widely used in the affected regions, providing a much-needed treatment option for patients suffering from trypanosomiasis. However, it is important to note that the drug may have certain side effects and precautions associated with its use, which should be carefully considered by healthcare professionals.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Melarsoprol: PKLR modulators and PKM2 modulators
PKLR modulators and PKM2 modulators are terms that refer to substances or compounds that can interact with and affect the activity of specific enzymes in the body.
From a biomedical perspective:
1. PKLR modulators: PKLR stands for pyruvate kinase liver and red blood cell. Pyruvate kinase is an enzyme involved in the final step of glycolysis, a metabolic pathway that converts glucose into energy. Modulators of PKLR can either enhance or inhibit the activity of this enzyme, thereby influencing the rate of glycolysis and energy production in the liver and red blood cells.
2. PKM2 modulators: PKM2 refers to pyruvate kinase muscle and tumor isoform 2. PKM2 is an enzyme that plays a role in glycolysis and is particularly abundant in cancer cells. Modulators of PKM2 can regulate its activity, potentially impacting the metabolic behavior of cancer cells and their ability to generate energy through glycolysis.
These terms indicate the potential therapeutic targets for drug development or research in the field of biomedicine. By modulating the activity of these enzymes, it may be possible to influence metabolic pathways and potentially treat conditions related to energy metabolism, such as certain types of cancer or metabolic disorders. However, without further context, it is challenging to provide more specific information about the significance or implications of PKLR and PKM2 modulation.
Drug Target R&D Trends for Melarsoprol
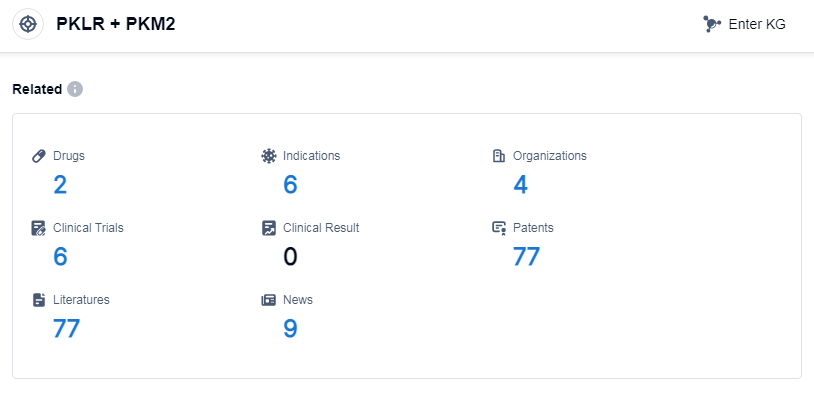
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 2 PKLR and PKM2 drugs worldwide, from 4 organizations, covering 6 indications, and conducting 6 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of the target PKLR and PKM2 indicates Sanofi as the leading company with an approved drug. The approved drug is indicated for trypanosomiasis. The development of a small molecule drug in the approved phase suggests progress in innovative drug development. France and the European Union have shown significant progress in the development of drugs under this target.
To fully understand the competitive landscape and future development of the target PKLR and PKM2, further analysis of the pipeline, ongoing research activities, and collaborations of the companies involved, as well as exploration of potential indications and drug types, would be necessary. Additionally, a more comprehensive analysis of countries/locations, including China, would provide a more complete picture of the global development of the target PKLR and PKM2.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Melarsoprol is a small molecule drug developed by Sanofi-Aventis France SA for the treatment of trypanosomiasis. Its approval in 1997 has significantly improved the treatment options for this infectious disease, offering hope to patients in affected regions. With its targeted approach and proven efficacy, Melarsoprol has become an important tool in the fight against trypanosomiasis.