Kyowa Kirin: Research on Mogamulizumab Targeting CCR4 in Chronic Neurodegenerative Diseases
Recently, Kyowa Kirin Co., Ltd., a Japanese pharmaceutical company, announced that it has signed a promotion and distribution agreement with NewBridge Pharmaceuticals for two drugs, aiming to cover the Middle East and North Africa (MENA) regions within its existing portfolio of rare disease products.
According to the terms of the agreement, NewBridge will have exclusive distribution rights in Algeria, Iraq, Libya, and Jordan for CRYSVITA® (burosumab) for the treatment of X-linked hypophosphatemia (XLH) and tumor-induced osteomalacia (TIO), as well as POTELIGEO® (mogamulizumab monoclonal antibody) for the treatment of two types of cutaneous T-cell lymphoma, including a rare form of non-Hodgkin lymphoma.
Mogamulizumab (Poteligeo) is a humanized, defucosylated, IgG1 monoclonal antibody specifically targeting CCR4. By binding to the N-terminal domain of CCR4, mogamulizumab induces antibody-dependent cellular cytotoxicity.
Burosumab is a monoclonal antibody specifically engineered to bind and neutralize the human protein fibroblast growth factor 23 (FGF23). In patients with X-linked hypophosphatemia (XLH), excessive FGF23 leads to low levels of phosphate in the blood, which adversely affects bone health. By inhibiting the action of FGF23, burosumab can increase phosphate levels in the blood, promote bone mineralization, and improve bone density and structure.
Regarding the CCR4 antibody: Mogamulizumab
Mogamulizumab was first approved in Japan in 2012 for the treatment of relapsed or refractory CCR4-positive adult T-cell leukemia/lymphoma (ATCL) and in 2014 for relapsed or refractory CCR4-positive cutaneous T-cell lymphoma (CTCL). CCR4 is a member of the chemokine receptor subfamily within the GPCRs (G-protein coupled receptors) family. By binding to the N-terminal domain of CCR4, mogamulizumab induces antibody-dependent cellular cytotoxicity (ADCC). The lack of fucosylation enhances the ADCC of mogamulizumab against CCR4-positive cells, offering a mechanistic advantage over traditionally glycosylated monoclonal antibodies.
In August and November of 2018, the FDA and EMA respectively approved Mogamulizumab for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy.
Mogamulizumab: New Clinical Indications
Beyond the classical targeting of CCR4 for inflammatory diseases and cancer therapy, researchers at Kyowa Kirin are exploring the use of mogamulizumab in the study of chronic neurodegenerative diseases.
Human T-cell leukemia virus type 1 (HTLV-1) associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic neurodegenerative disease. HAM/TSP was first described in the 19th century in Jamaica, but it wasn't until the 1980s that the cause of the disease was identified as the HTLV-1 retrovirus infection. HAM/TSP leads to chronic disability and thus imposes a significant public health burden in areas where HTLV-1 infection is endemic. Due to the lack of accurate animal models, research progress has been limited. Additionally, the treatment for HAM/TSP remains highly unsatisfactory: Antiretroviral drugs have minimal impact on the infection, and although potential disease-modifying therapies are widely used, their effectiveness has not yet been confirmed.
Previous studies have shown that in both patients with HAM/TSP and HTLV-1 carriers, there is a high rate of HTLV-1 infection in CCR4 positive T cells.
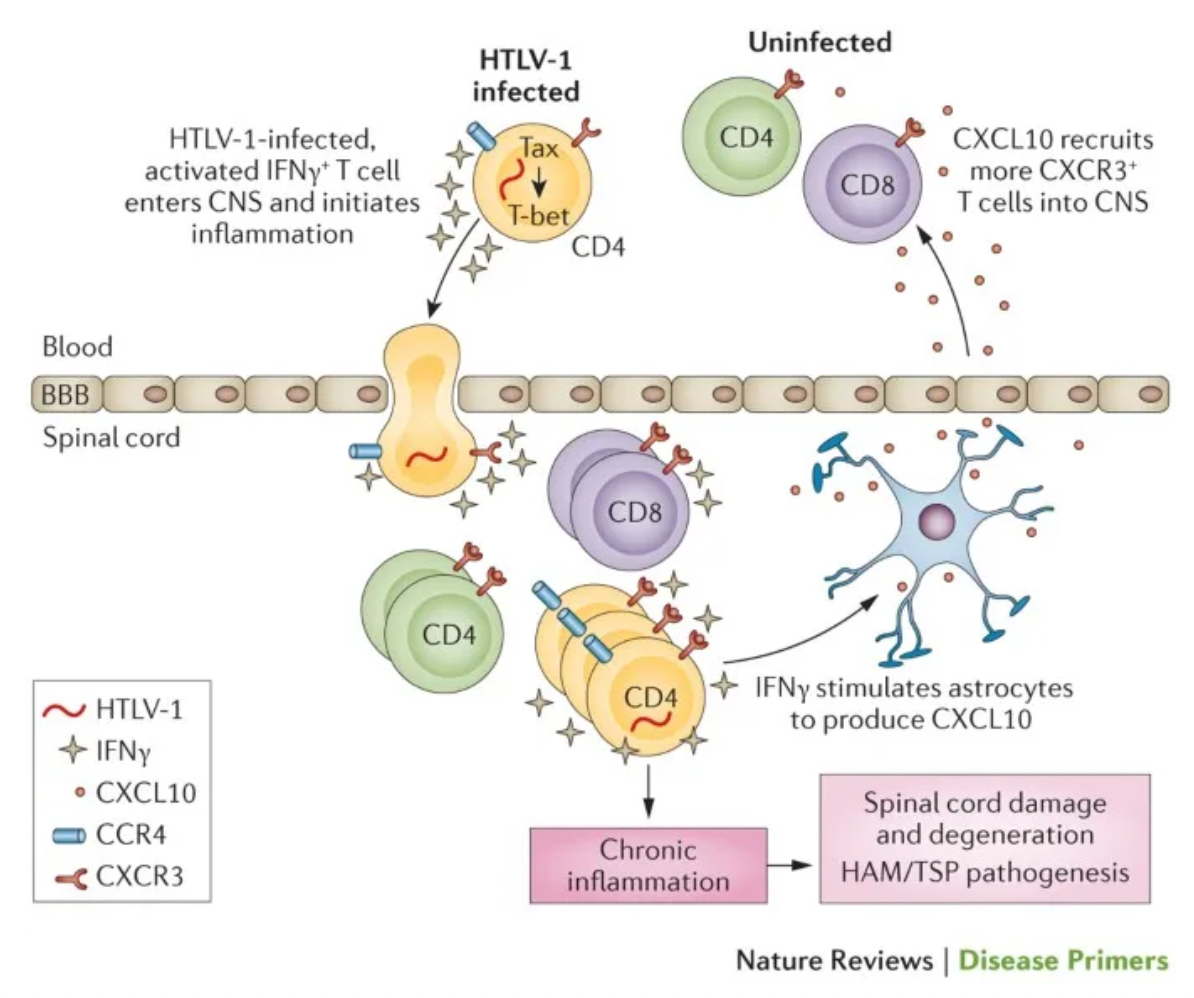
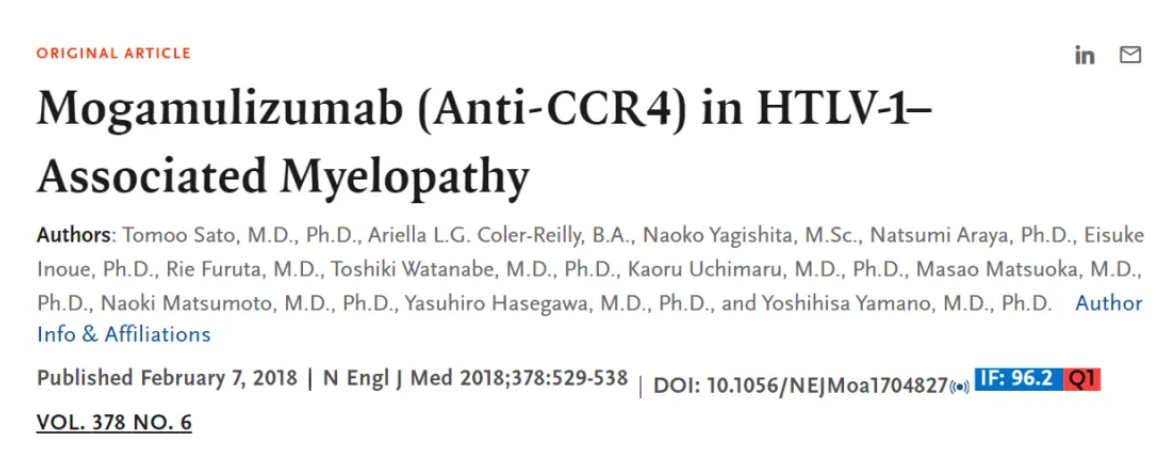
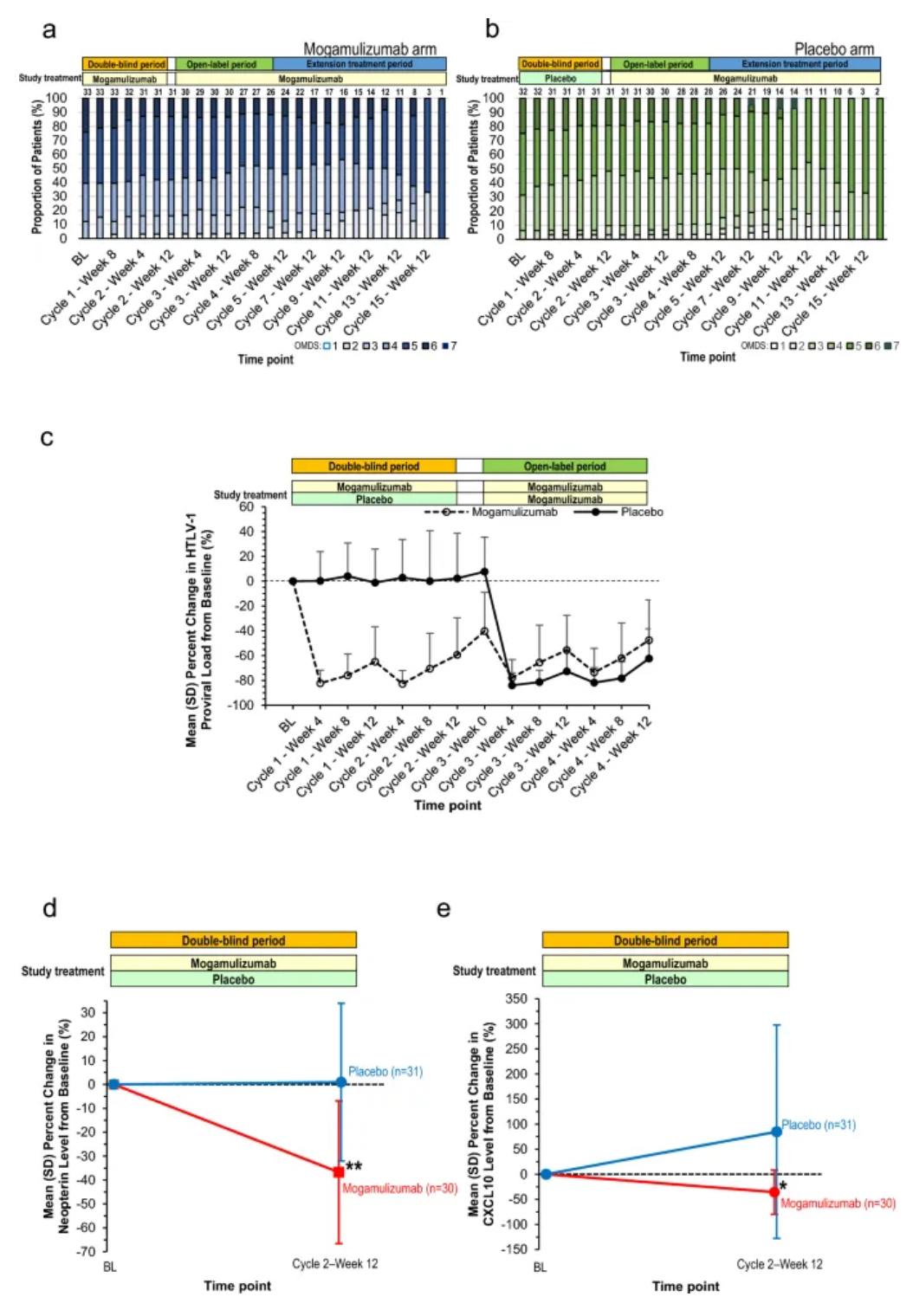
Therefore, researchers initiated a Phase I/IIa clinical trial for this indication, along with a study on the long-term safety and efficacy of this monoclonal antibody. The results showed that the monoclonal antibody mogamulizumab reduced the viral load of the original virus in peripheral blood in a dose-dependent manner, decreased levels of the inflammatory marker CXCL10 (C-X-C motif chemokine ligand 10) and methotrexate in cerebrospinal fluid (CSF), and improved clinical symptoms such as spasticity and movement disorders. These findings were published in the New England Journal of Medicine in 2018.
In updated clinical study results from 2024, at the end of the double-blind period, the mogamulizumab monoclonal antibody group showed a significant reduction in HTLV-1 original viral load (-59.39±29.91% vs. placebo group 2.32±36.31%) and a significant decrease in levels of methotrexate (p < 0.001) and CXCL10 (p = 0.004) in CSF. Although no significant differences were observed in clinical benefits (the primary endpoint was the proportion of patients with an improvement of ≥1 grade in the Osame Motor Disability Score (OMDS)), mogamulizumab monoclonal antibody may provide long-term clinical benefits by preventing disease progression.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!