Turnstone Biologics Reports Promising Initial Findings from Phase 1 TIDAL-01 Study in Advanced Colorectal Cancer
Turnstone Biologics Corp. ("Turnstone" or the "Company") (Nasdaq: TSBX), a clinical-stage biotechnology firm focused on advancing innovative treatments for solid tumors through its pioneering selected tumor-infiltrating lymphocyte (Selected TIL) therapy, announced promising early results from its Phase 1 STARLING trial of TIDAL-01 in treating metastatic metastatic microsatellite stable colorectal cancer ("MSS mCRC").
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The Phase 1 STARLING trial conducted by Turnstone is a multi-center, initial-in-human, non-randomized, open label, single-dose investigation, assessing the safety, tolerability, and clinical activity of TIDAL-01. Current recruitment targets patients with colorectal cancer, head and neck squamous cell carcinoma, and uveal melanoma. As observed from initial data up to July 15, 2024, important conclusions include:
The Phase 1 STARLING trial conducted by Turnstone is a multi-center, initial-in-human, non-randomized, open label, single-dose investigation, assessing the safety, tolerability, and clinical activity of TIDAL-01. Current recruitment targets patients with colorectal cancer, head and neck squamous cell carcinoma, and uveal melanoma. As observed from initial data up to July 15, 2024, important conclusions include:
Clinical Responses: Out of the four evaluable patients with MSS mCRC, a 25% overall response rate (ORR) and a 50% disease control rate (DCR) were noted. One individual exhibited a profound and durable ongoing complete response (CR).
Durability of Response: Half of the participants showcased sustained clinical benefit; notably, the patient with ongoing CR experienced progression-free survival extending beyond one year, and another with stable disease evidenced six months of progression-free survival.
Translational Profile: The TIDAL-01 process effectively generated high titer, polyclonal and multi-epitope tumor neoantigen-reactive T cells, which expanded in the patient, persisted in the blood, and were linked with increased CD8 T cell tumor infiltration.
Tolerability Profile: TIDAL-01 demonstrated good tolerability. Safety events observed were consistent with known adverse events related to the lymphodepletion treatment, and the administration of IL-2 and pembrolizumab.
Manufacturing Rates: An 80% manufacturing success rate was achieved for TIDAL-01 in CRC patients with adequate starting material, aligning with other early-stage cell therapy processes. The target dose of at least 1×10⁹ total T cells was surpassed in all CRC products manufactured.
"The promising preliminary clinical outcomes from the Phase 1 STARLING trial underscore TIDAL-01's potential in redefining treatment for metastatic CRC and other solid tumors,” stated Sammy Farah, M.B.A., Ph.D., President and CEO of Turnstone. “Achieving a 25% ORR with a significant and enduring response, plus a 50% DCR, presents a favorable comparison to current therapies showing a 1-6% ORR and median progression-free survival (mPFS) of 2.0-5.6 months. Combined with a well-tolerated profile and manufacturing robustness, these results solidify our commitment to advancing TIDAL-01 for patients facing substantial treatment gaps. We are extremely optimistic about the positive initiation of our clinical research."
"CRC is a significant cause of cancer-related mortality in the U.S.," remarked Mike Burgess, MBChB, Ph.D., Interim Chief Medical Officer of Turnstone. "Early indications of clinical benefit reveal TIDAL-01 as a potentially viable therapeutic option for metastatic CRC. We are pleased to report that the patient with a complete response is still in remission over a year post-treatment. The STARLING study will continue to explore the effectiveness of TIDAL-01 in CRC and other tumors with limited treatments, such as uveal melanoma and head and neck cancer. We are eager to advance pioneering treatments that enhance clinical efficacy and address paramount needs in oncology.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
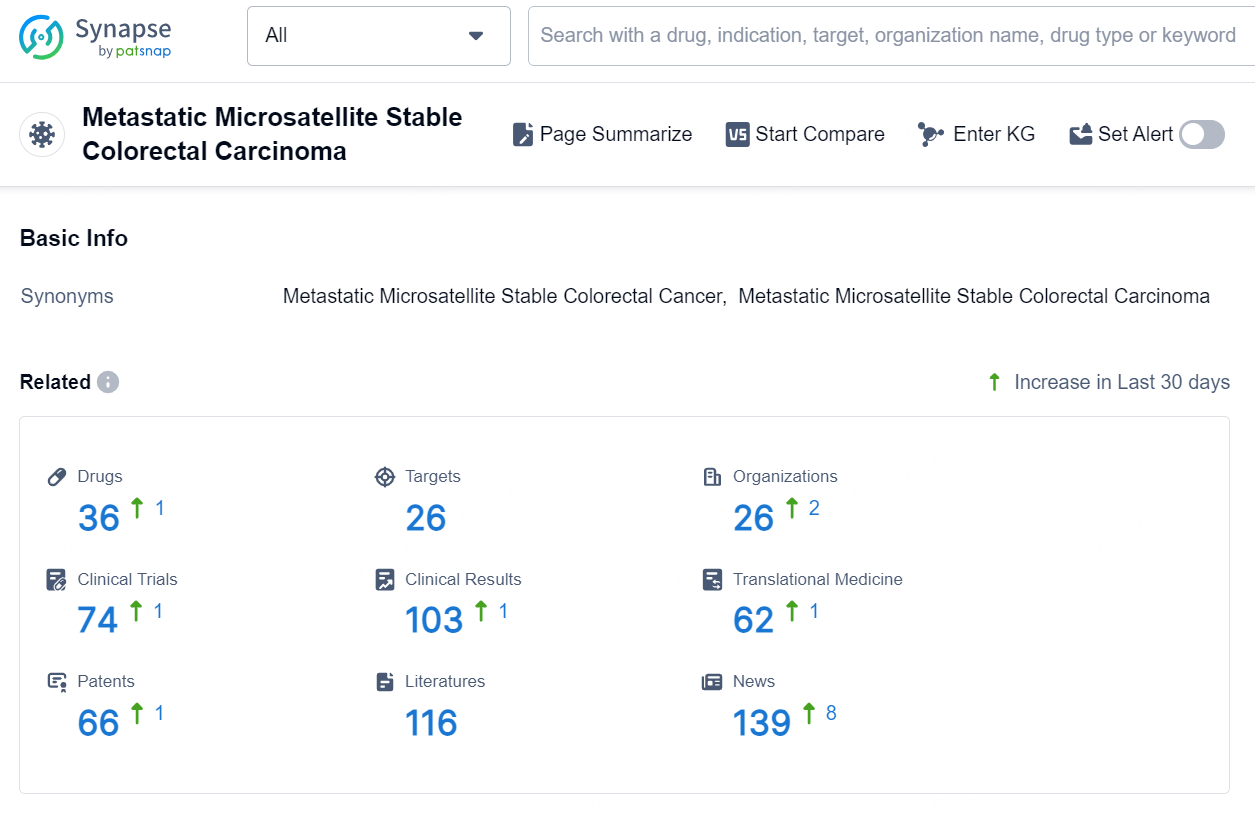
According to the data provided by the Synapse Database, As of August 20, 2024, there are 36 investigational drugs for the Metastatic Microsatellite Stable Colorectal Carcinoma, including 26 targets, 26 R&D institutions involved, with related clinical trials reaching 74, and as many as 66 patents.
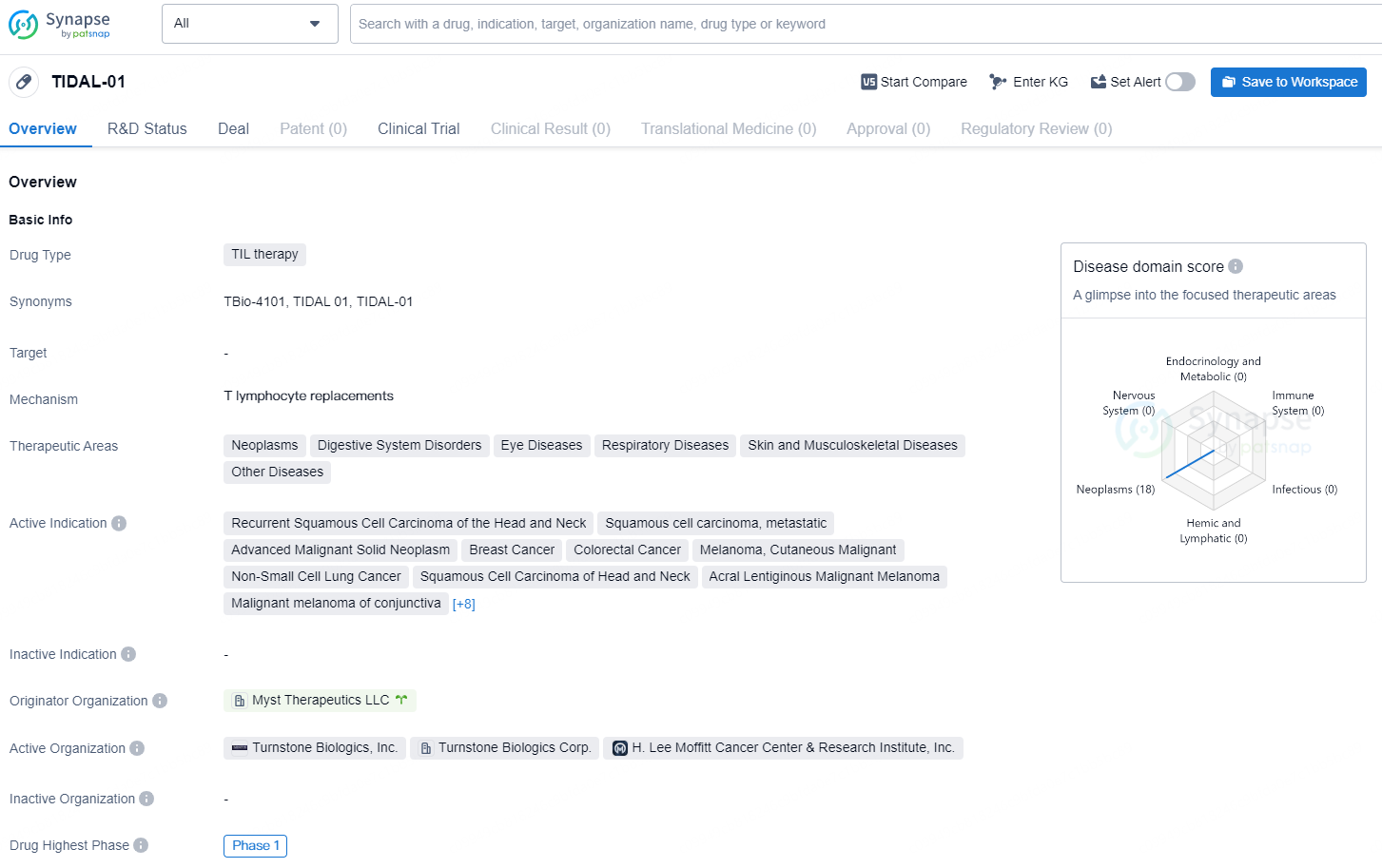
TIDAL-01, Turnstone’s lead Selected TIL therapy candidate, utilizes an unbiased identification and functional screening process to isolate and selectively expand the most comprehensive set of tumor-reactive TILs from the patient’s tumor. For more effective tumor killing, the TIDAL-01 production process targets a product with a significantly higher proportion of functional and potent tumor-reactive T cells compared to bulk TIL and is also designed to deliver at least 10⁹ cells. TIDAL-01 is currently advancing in ongoing multi-site, first-in-human, non-randomized, open-label, single-dose study Phase 1 trials, which include the Company sponsored STARLING trials and the investigator sponsored trials with Moffitt Cancer Center. The Phase 1 studies are evaluating safety, biology, initial efficacy, and manufacturing feasibility of TIDAL-01 in patients with solid tumors where standard bulk TILs have historically not shown objective and/or durable responses in clinical trials.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!